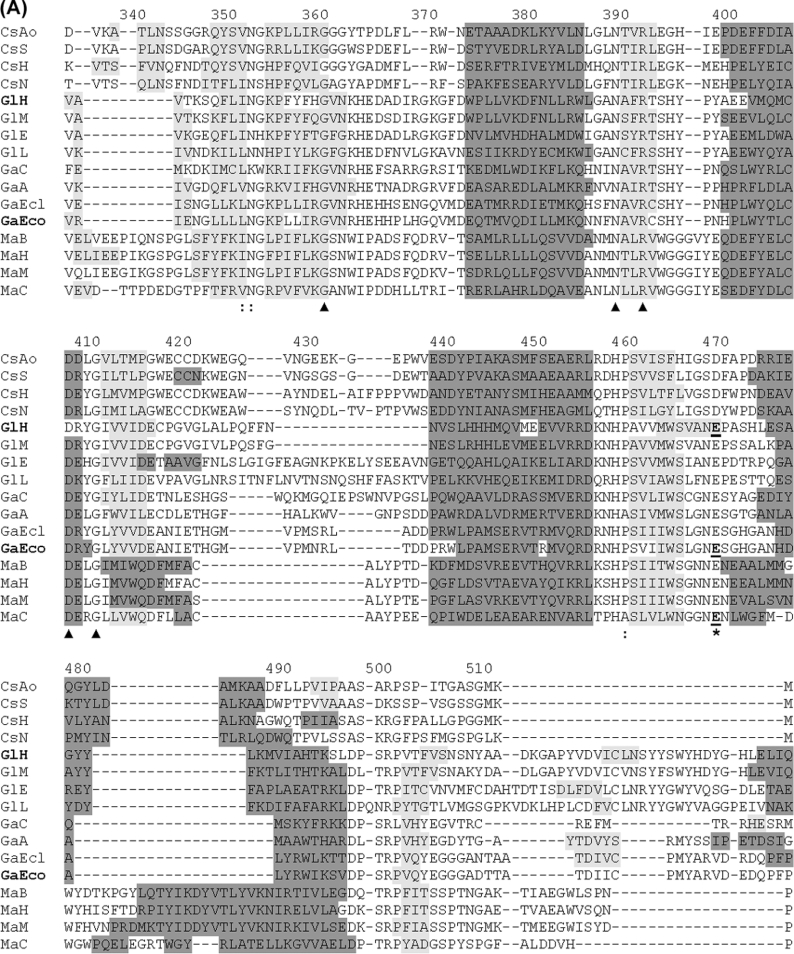
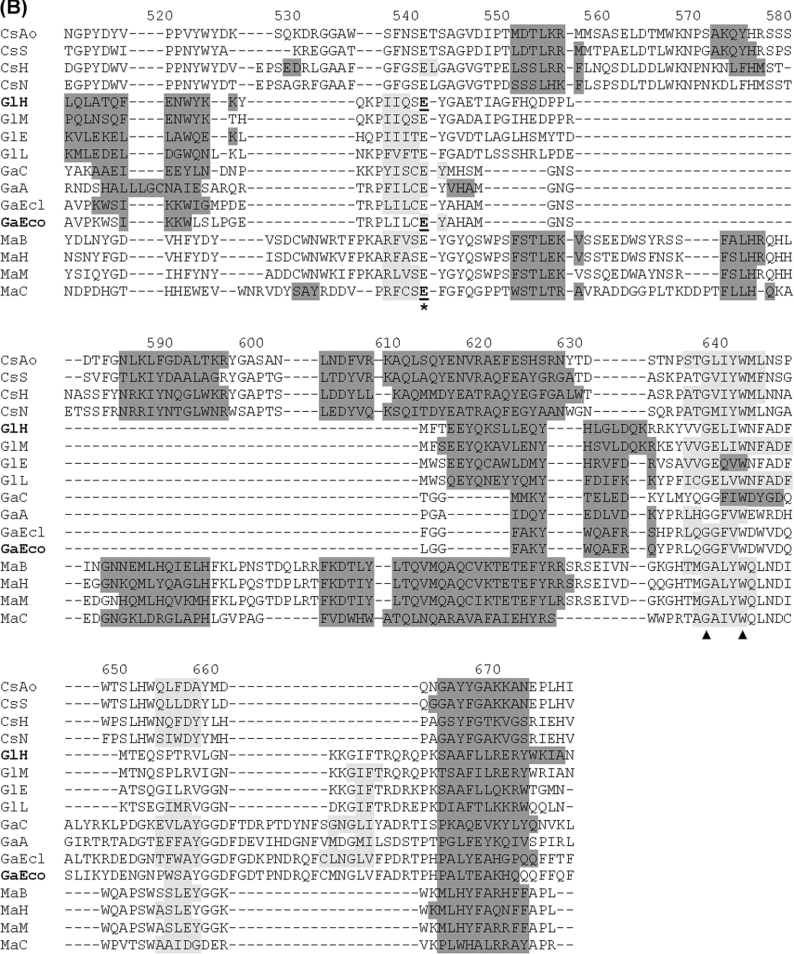
Figure 7. Assignment of putative catalytic residues in CsxA and related ORFs through structure-guided alignment of TIM-barrel domains from a representative set of GH2 sequences.
Abbreviations are as in Figure 6. Alignment was obtained by the 3DCoffee structure-guided-alignment method [20] using domain 3 from human β-glucuronidase as template. Secondary structures are shaded in dark grey (α-helices) or light grey (β-sheets). Secondary structures formally identified in crystallized proteins are shown for human β-glucuronidase (PDB ID: 1bhg:A) and E. coli β-galactosidase LacZ (PDB ID: 1DPO:A). These two proteins are indicated by bold labels GlH and GaEco respectively. Secondary structures in other proteins were predicted by the PSIPRED algorithm [21]. The putative catalytic acid/base and nucleophile residues are indicated with asterisks. Catalytic residues formally identified by mechanistic studies [35–39] are underlined. Other strictly conserved residues are indicated with triangles. Numbering refers to the primary structure of CsxA (as in Figure 4).