Abstract
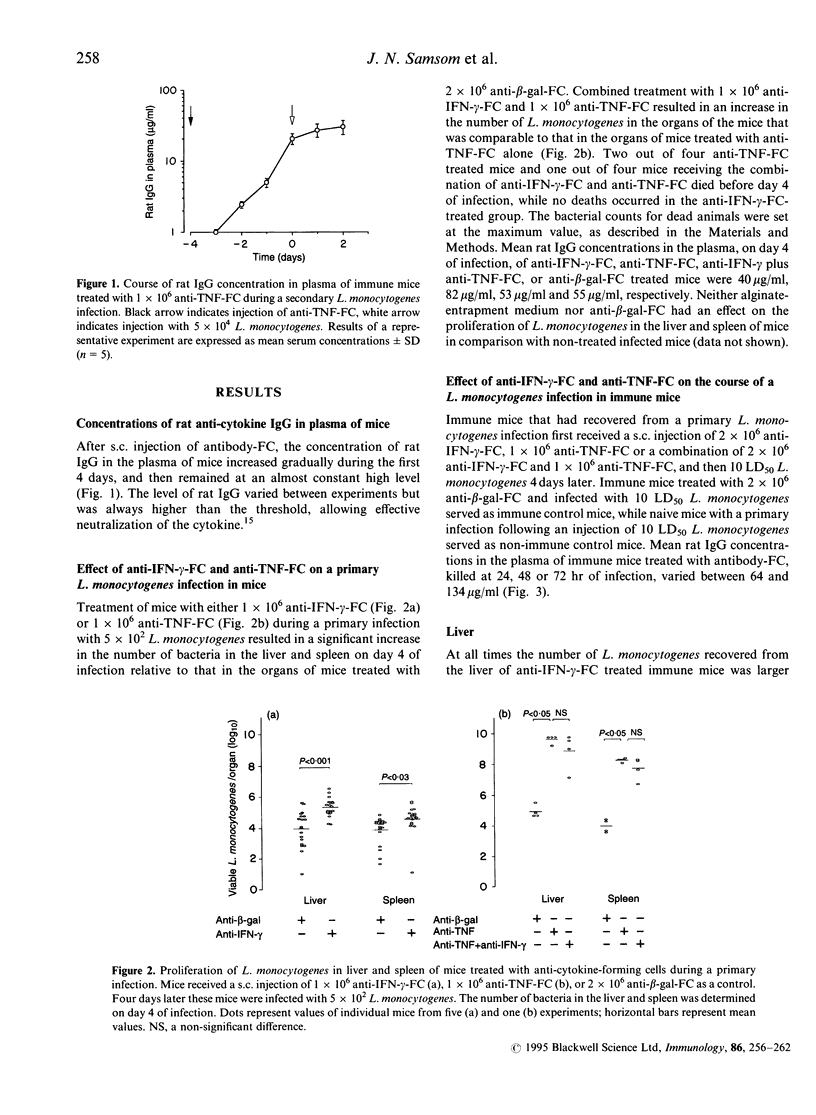
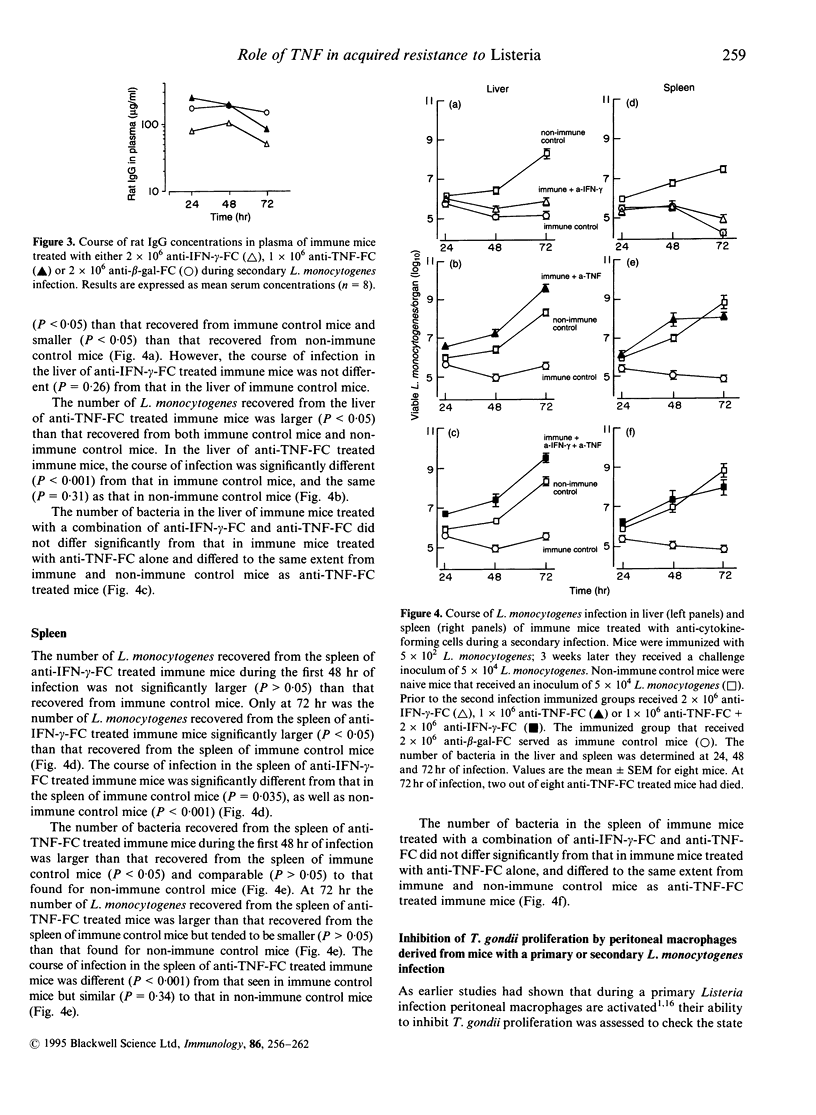
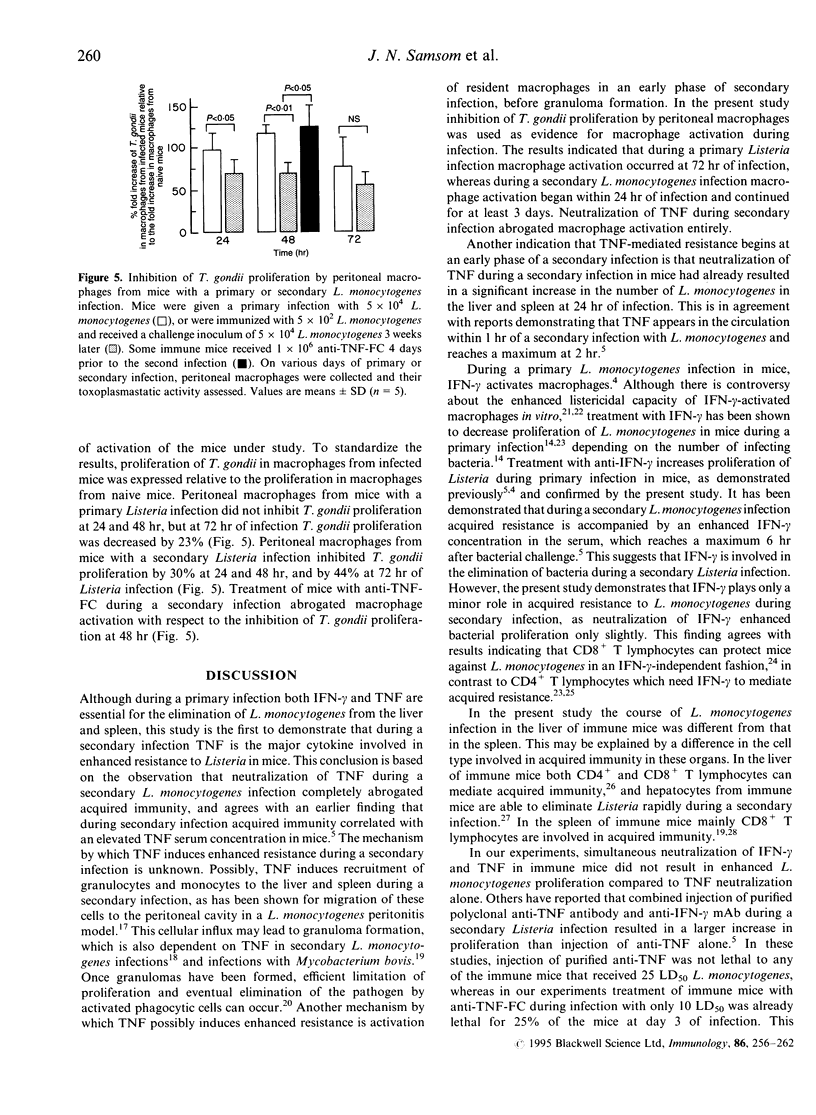
Mice with a secondary Listeria monocytogenes infection eliminate the bacteria much faster and more efficiently from their organs than mice with a primary infection. During the course of a secondary infection, serum concentrations of interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF) are higher than during a primary infection. The aim of the present study was to determine whether these cytokines are involved in the acquired resistance to L. monocytogenes during a secondary infection in mice. In order to neutralize cytokines, alginate-encapsulated cells, which form anti-cytokine monoclonal antibodies, were injected into the nuchal region of mice during a Listeria infection. Mice recovered from a sublethal primary Listeria infection, which acquired cell-mediated immunity, received a subcutaneous injection of anti-IFN-gamma-forming cells, or anti-TNF-forming cells, and 4 days later received an intravenous injection with 10 50% lethal dose (LD50) L. monocytogenes. The number of bacteria recovered from the liver and spleen of immune mice treated with anti-IFN-gamma-forming cells was slightly larger (approximately 1 log10) than that found for immune mice treated with anti-beta-galactosidase-forming cells, called immune control mice. The organs of immune mice treated with anti-TNF-forming cells yielded significantly more (approximately 4 log10) bacteria than those of immune control mice, more than those of immune mice treated with anti-IFN-gamma-forming cells, and comparable numbers to those of non-immune mice. Taken together, these results demonstrate that TNF is essential in acquired resistance to L. monocytogenes during a secondary infection in mice, while IFN-gamma plays a minor role.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Roncarolo M. G., Yssel H., Andersson U., Gleich G. J., Silver J. E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992 Jun;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. A., Canono B. P., Cook J. L. Mouse macrophages stimulated by recombinant gamma interferon to kill tumor cells are not bactericidal for the facultative intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 May;56(5):1371–1375. doi: 10.1128/iai.56.5.1371-1375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S. H., Barczynski L. K., Wing E. J. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J Leukoc Biol. 1992 Apr;51(4):421–424. doi: 10.1002/jlb.51.4.421. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Harty J. T., Schreiber R. D., Bevan M. J. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Activation of mouse peritoneal macrophages during infection with Salmonella typhimurium does not result in enhanced intracellular killing. J Immunol. 1990 Jun 1;144(11):4340–4346. [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Endogenous tumor necrosis factor alpha is required for enhanced antimicrobial activity against Toxoplasma gondii and Listeria monocytogenes in recombinant gamma interferon-treated mice. Infect Immun. 1992 Dec;60(12):5107–5112. doi: 10.1128/iai.60.12.5107-5112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van der Meide P. H., van Furth R. Intravenous injection of interferon-gamma inhibits the proliferation of Listeria monocytogenes in the liver but not in the spleen and peritoneal cavity. Immunology. 1992 Nov;77(3):354–361. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-gamma. J Immunol. 1988 Nov 1;141(9):3203–3207. [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Rosen H., Brocke S., Peters C., Hahn H. Protective immunity and granuloma formation are mediated by two distinct tumor necrosis factor alpha- and gamma interferon-dependent T cell-phagocyte interactions in murine listeriosis: dissociation on the basis of phagocyte adhesion mechanisms. Infect Immun. 1992 May;60(5):1875–1882. doi: 10.1128/iai.60.5.1875-1882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kohanawa M., Chen Y., Sato H., Moriyama M., Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989 Nov;57(11):3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhmilevich A. L. Evidence for a significant role of CD4+ T cells in adoptive immunity to Listeria monocytogenes in the liver. Immunology. 1994 Jun;82(2):249–254. [PMC free article] [PubMed] [Google Scholar]
- Rothe J., Lesslauer W., Lötscher H., Lang Y., Koebel P., Köntgen F., Althage A., Zinkernagel R., Steinmetz M., Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993 Aug 26;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Savelkoul H. F., Seymour B. W., Sullivan L., Coffman R. L. IL-4 can correct defective IgE production in SJA/9 mice. J Immunol. 1991 Mar 15;146(6):1801–1805. [PubMed] [Google Scholar]
- Savelkoul H. F., van Ommen R., Vossen A. C., Breedland E. G., Coffman R. L., van Oudenaren A. Modulation of systemic cytokine levels by implantation of alginate encapsulated cells. J Immunol Methods. 1994 Apr 15;170(2):185–196. doi: 10.1016/0022-1759(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Stewart J. R., Blackwell W. H., Crute S. L., Loughlin V., Greenfield L. J., Hess M. L. Inhibition of surgically induced ischemia/reperfusion injury by oxygen free radical scavengers. J Thorac Cardiovasc Surg. 1983 Aug;86(2):262–272. [PubMed] [Google Scholar]
- Stewart J. R., Blackwell W. H., Crute S. L., Loughlin V., Greenfield L. J., Hess M. L. Inhibition of surgically induced ischemia/reperfusion injury by oxygen free radical scavengers. J Thorac Cardiovasc Surg. 1983 Aug;86(2):262–272. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., van den Barselaar M. T., Sluiter W., Leijh P. C., van Furth R. Divergent changes in antimicrobial activity after immunologic activation of mouse peritoneal macrophages. J Immunol. 1987 Sep 1;139(5):1665–1672. [PubMed] [Google Scholar]
- van Furth R., van Zwet T. L., Buisman A. M., van Dissel J. T. Anti-tumor necrosis factor antibodies inhibit the influx of granulocytes and monocytes into an inflammatory exudate and enhance the growth of Listeria monocytogenes in various organs. J Infect Dis. 1994 Jul;170(1):234–237. doi: 10.1093/infdis/170.1.234. [DOI] [PubMed] [Google Scholar]
- van Ommen R., Vredendaal A. E., Savelkoul H. F. Suppression of polyclonal and antigen-specific murine IgG1 but not IgE responses by neutralizing interleukin-6 in vivo. Eur J Immunol. 1994 Jun;24(6):1396–1403. doi: 10.1002/eji.1830240624. [DOI] [PubMed] [Google Scholar]


