Abstract
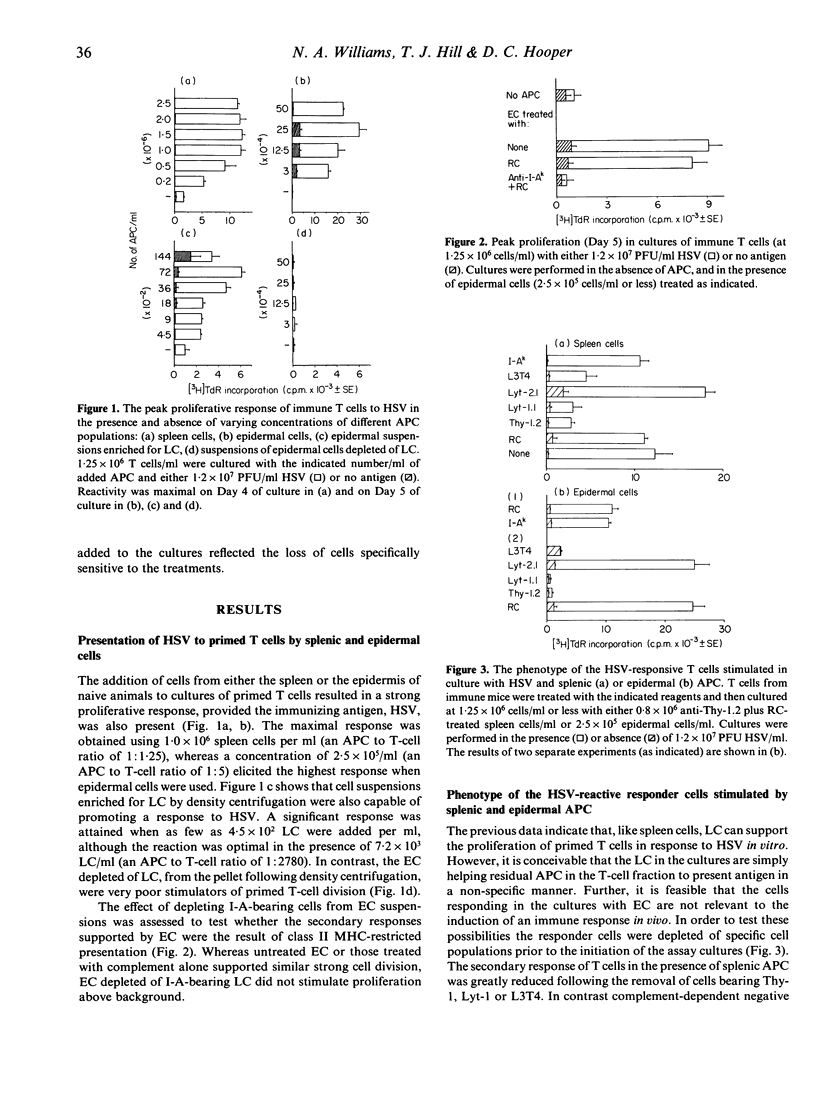
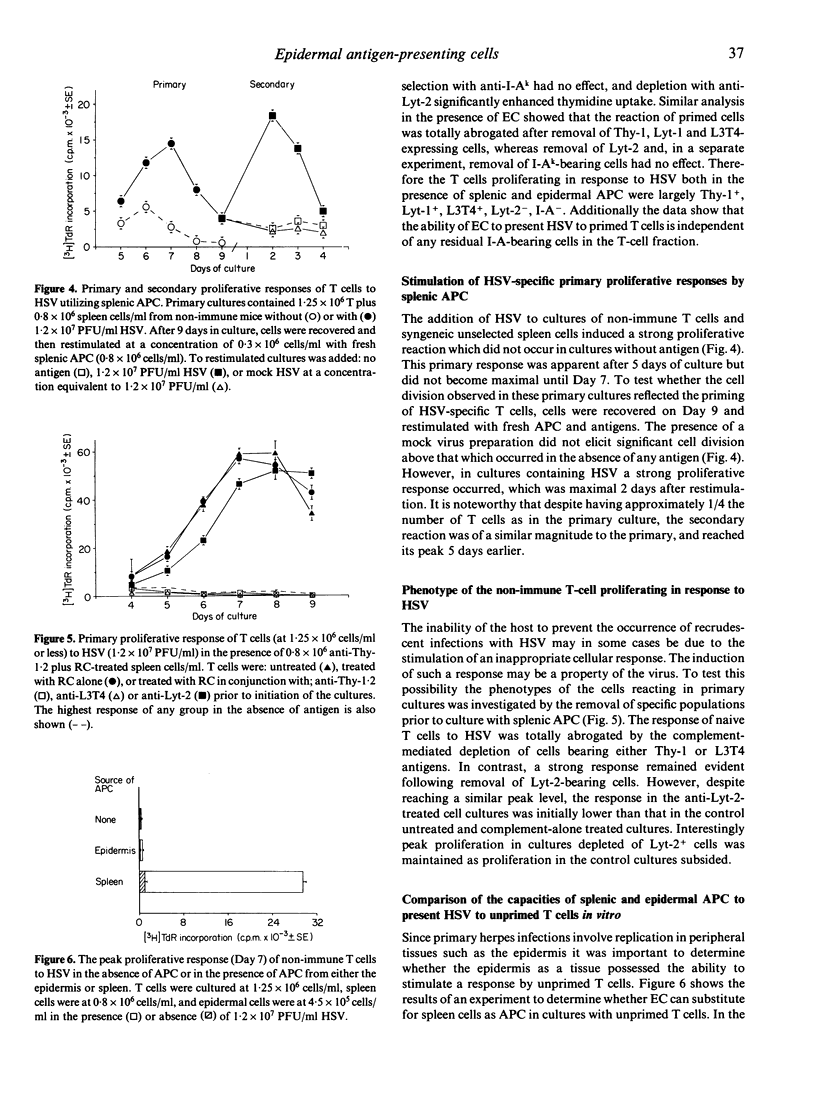
The role of epidermal Langerhans' cells in infection with herpes simplex virus (HSV) was investigated using a culture system that supports antigen-specific primary and secondary T-cell proliferative responses. Epidermal cell suspensions were capable of restimulating the response of in vivo primed T cells to UV-inactivated HSV. This capability was also present in cell suspensions enriched for Langerhans' cells, but was abrogated by the depletion of I-A-bearing cells. The magnitude, kinetics and phenotype of the responding cells were similar to those elicited when HSV was presented to primed T cells by antigen-presenting cells from the spleen. In marked contrast, whereas splenic antigen-presenting cells induced strong antigen-specific proliferation of unprimed T cells (primarily of the helper phenotype), Langerhans' cells failed to invoke any detectable reaction of such cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Braathen L. R., Berle E., Mobech-Hanssen U., Thorsby E. Studies on human epidermal Langerhans' cells: II. Activation of human T lymphocytes to herpes simplex virus. Acta Derm Venereol. 1980;60(5):381–387. [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Inaba K., Schuler G., Witmer M. D., Valinksy J., Atassi B., Steinman R. M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986 Aug 1;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., Bowers W. E. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982 Jul 1;156(1):1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Bedford P., Hunt R. The role of dendritic cells in the initiation of immune responses to contact sensitizers. II. Studies in nude mice. Cell Immunol. 1985 Sep;94(2):435–439. doi: 10.1016/0008-8749(85)90267-9. [DOI] [PubMed] [Google Scholar]
- Lassila O., Vainio O., Matzinger P. Can B cells turn on virgin T cells? Nature. 1988 Jul 21;334(6179):253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. The MRC OX-22- CD4+ T cells that help B cells in secondary immune responses derive from naive precursors with the MRC OX-22+ CD4+ phenotype. J Exp Med. 1989 Mar 1;169(3):653–662. doi: 10.1084/jem.169.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. Development of restrictions in the expression of immunoglobulin specificities by lymphoid cells. Transplant Rev. 1970;5:19–44. doi: 10.1111/j.1600-065x.1970.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Shelley W. B., Juhlin L. Langerhans cells form a reticuloepithelial trap for external contact antigens. Nature. 1976 May 6;261(5555):46–47. doi: 10.1038/261046a0. [DOI] [PubMed] [Google Scholar]
- Shelley W. B., Juhlin L. Selective uptake of contact allergens by the Langerhans cell. Arch Dermatol. 1977 Feb;113(2):187–192. [PubMed] [Google Scholar]
- Shevach E. M., Paul W. E., Green I. Histocompatibility-linked immune response gene function in guinea pigs. Specific inhibition of antigen-induced lymphocyte proliferation by alloantisera. J Exp Med. 1972 Nov 1;136(5):1207–1221. doi: 10.1084/jem.136.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg I., Baer R. L., Rosenthal S. A. The role of langerhans cells in contact allergy. I. An ultrastructural study in actively induced contact dermatitis in guinea pigs. Acta Derm Venereol. 1974;54(5):321–331. [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stingl G., Gazze-Stingl L. A., Aberer W., Wolff K. Antigen presentation by murine epidermal langerhans cells and its alteration by ultraviolet B light. J Immunol. 1981 Oct;127(4):1707–1713. [PubMed] [Google Scholar]
- Stingl G., Katz S. I., Shevach E. M., Wolff-Schreiner E., Green I. Detection of Ia antigens on Langerhans cells in guinea pig skin. J Immunol. 1978 Feb;120(2):570–578. [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. Biochemistry and biology of antigen presentation by macrophages. Cell Immunol. 1986 Apr 15;99(1):3–6. doi: 10.1016/0008-8749(86)90208-x. [DOI] [PubMed] [Google Scholar]
- Williams N. A., Hill T. J., Hooper D. C. Murine epidermal antigen-presenting cells in primary and secondary T-cell proliferative responses to a soluble protein antigen in vitro. Immunology. 1990 Nov;71(3):411–416. [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S., Okabe N., Mori R. Role of epidermal Langerhans cells in resistance to herpes simplex virus infection. Arch Virol. 1986;90(3-4):261–271. doi: 10.1007/BF01317375. [DOI] [PubMed] [Google Scholar]


