Abstract
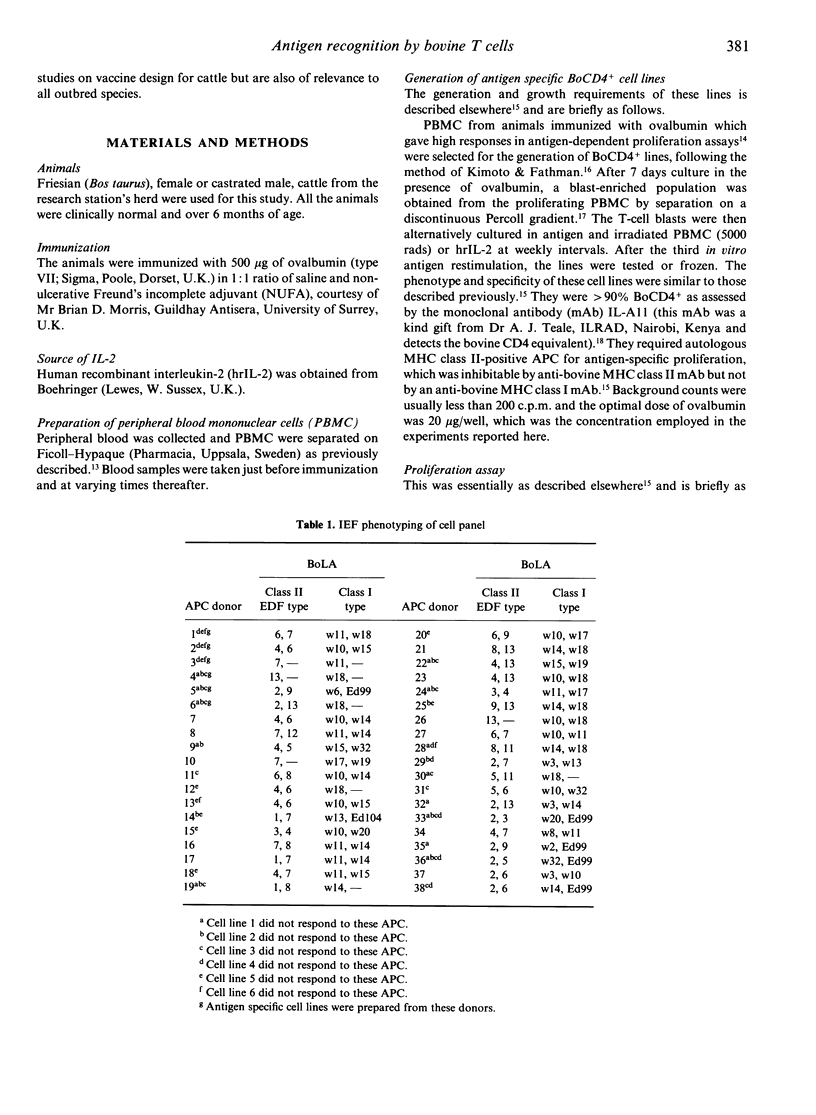
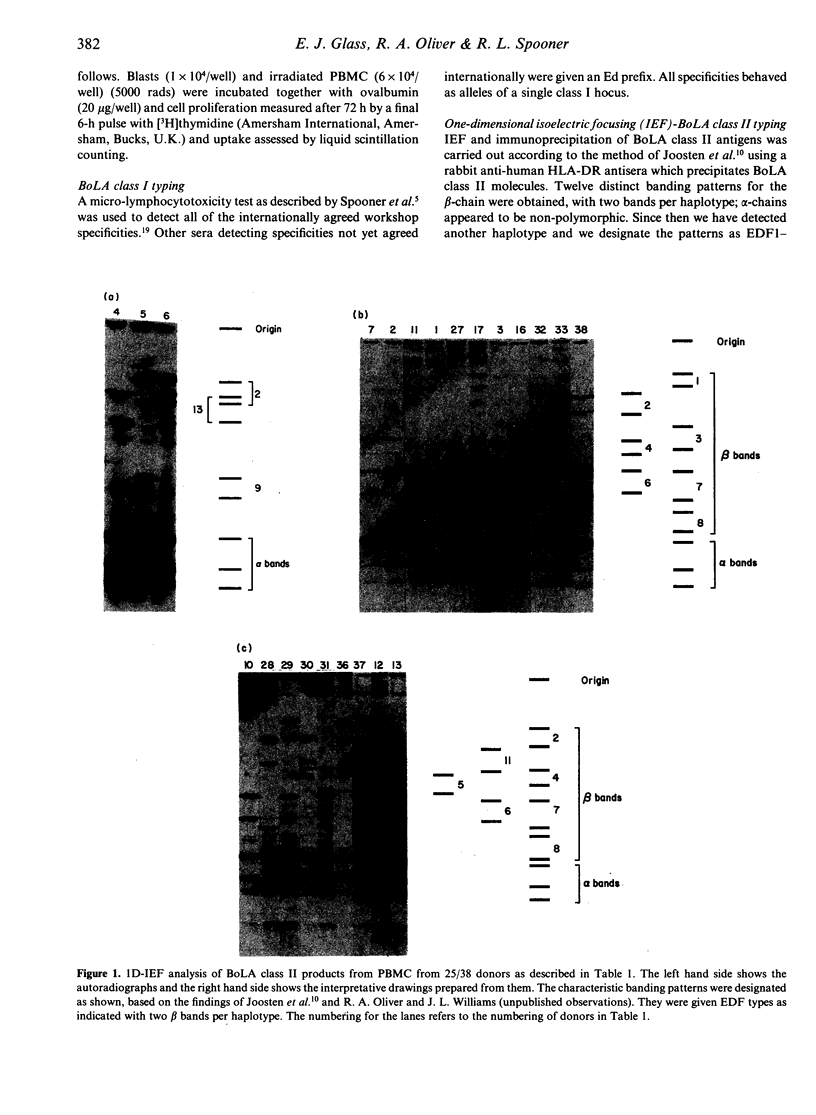
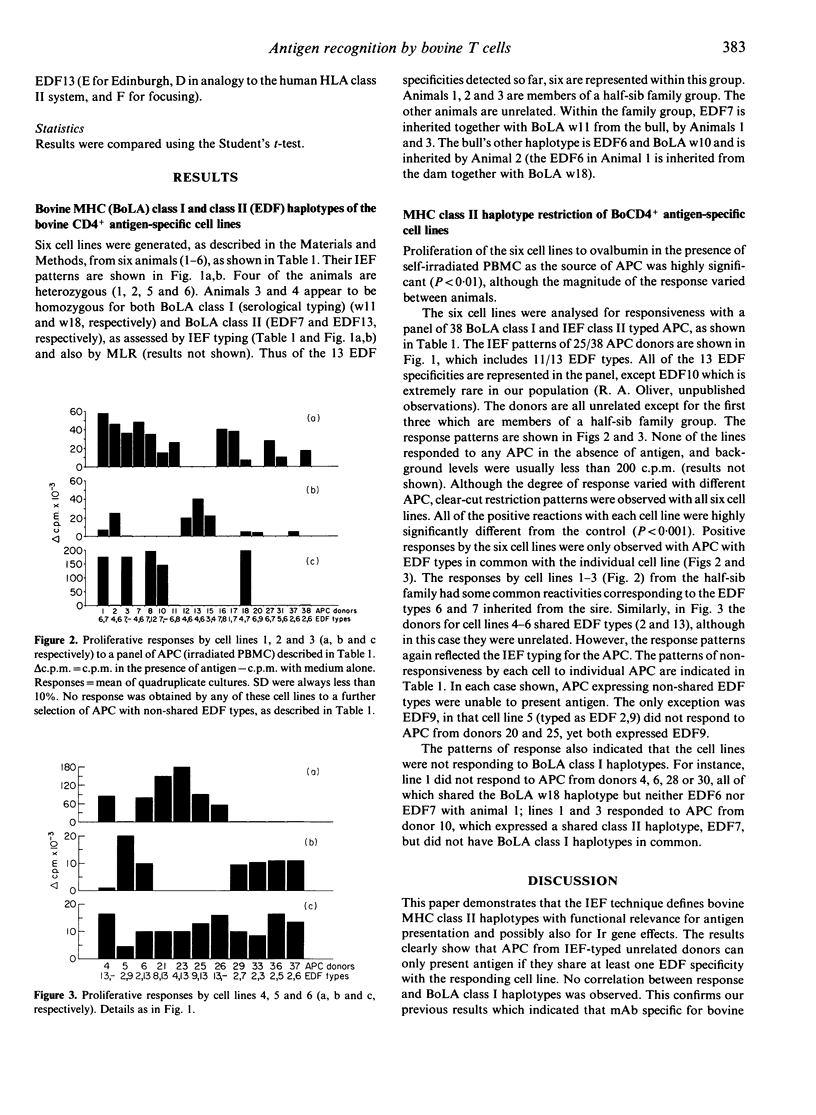
A recently established, one-dimensional isoelectric focusing (IEF) method for distinguishing major histocompatibility complex (MHC) class II polymorphisms in an outbred species, cattle, has allowed us to analyse the involvement of the MHC in the recognition of antigen by bovine T cells. Bovine T-cell lines of Th cell phenotype (BoCD4+) specific for ovalbumin were generated from six individual high responder animals. These animals were bovine MHC (BoLA) class II typed using the IEF technique which detects bovine DR-like products. Four of the animals were shown to be heterozygous and two were homozygous for the IEF specificities. Six out of the 13 IEF specificities (EDF types) detected so far were represented by this group of animals. The cell lines were tested against a panel of IEF-typed antigen-presenting cells (APC) from unrelated donors. The lines only responded to antigen in proliferation assays when the APC shared at least one MHC class II EDF specificity with the BoCD4+ cell line. The responses did not correlate with BoLA class I specificities. However, lines from one of the animals were consistently generated to one of the two haplotypes only. This suggests that there are non-responder alleles to a multi-epitope antigen, present in the cattle population. The results demonstrate that IEF of bovine MHC class II products defines haplotypes of functional relevance, and may indeed be identifying the actual restriction elements involved in presentation of ovalbumin. These results have important implications for future vaccine design in an outbred species, particularly in terms of immune response gene effects and disease associations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L., Böhme J., Rask L., Peterson P. A. Genomic hybridization of bovine class II major histocompatibility genes: 1. Extensive polymorphism of DQ alpha and DQ beta genes. Anim Genet. 1986;17(2):95–112. doi: 10.1111/j.1365-2052.1986.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Baldwin C. L., Teale A. J., Naessens J. G., Goddeeris B. M., MacHugh N. D., Morrison W. I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986 Jun 15;136(12):4385–4391. [PubMed] [Google Scholar]
- Bergholtz B. O., Thorsby E. HLA-D restriction of the macrophage-dependent response of immune human T lymphocytes to PPD in vitro: inhibition by anti-HLA-DR antisera. Scand J Immunol. 1978;8(1):63–73. doi: 10.1111/j.1365-3083.1978.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Bujdoso R., Young P., Harkiss G. D., McConnell I. Antigen presentation in the sheep: generation of antigen-specific T-cell lines. Immunology. 1989 Apr;66(4):559–564. [PMC free article] [PubMed] [Google Scholar]
- Bull R. W., Lewin H. A., Wu M. C., Peterbaugh K., Antczak D., Bernoco D., Cwik S., Dam L., Davies C., Dawkins R. L. Joint report of the Third International Bovine Lymphocyte Antigen (BoLA) Workshop, Helsinki, Finland, 27 July 1986. Anim Genet. 1989;20(1):109–132. doi: 10.1111/j.1365-2052.1989.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Glass E. J., Oliver R. A., Spooner R. L. Variation in T cell responses to ovalbumin in cattle: evidence for Ir gene control. Anim Genet. 1990;21(1):15–28. doi: 10.1111/j.1365-2052.1990.tb03203.x. [DOI] [PubMed] [Google Scholar]
- Glass E. J., Spooner R. L. Generation and characterisation of bovine antigen-specific T cell lines. J Immunol Methods. 1990 Apr 17;128(2):267–275. doi: 10.1016/0022-1759(90)90219-l. [DOI] [PubMed] [Google Scholar]
- Glass E. J., Spooner R. L. Requirement for MHC class II positive accessory cells in an antigen specific bovine T cell response. Res Vet Sci. 1989 Mar;46(2):196–201. [PubMed] [Google Scholar]
- Groenen M. A., van der Poel J. J., Dijkhof R. J., Giphart M. J. The nucleotide sequence of bovine MHC class II DQB and DRB genes. Immunogenetics. 1990;31(1):37–44. doi: 10.1007/BF00702487. [DOI] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Buus S., Sette A., Grey H. M., Smith J. A., Gefter M. L. Immunological self, nonself discrimination. Science. 1987 Feb 20;235(4791):865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- Joosten I., Hensen E. J., Sanders M. F., Andersson L. Bovine MHC class II restriction fragment length polymorphism linked to expressed polymorphism. Immunogenetics. 1990;31(2):123–126. doi: 10.1007/BF00661223. [DOI] [PubMed] [Google Scholar]
- Joosten I., Sanders M. F., van der Poel A., Williams J. L., Hepkema B. G., Hensen E. J. Biochemically defined polymorphism of bovine MHC class II antigens. Immunogenetics. 1989;29(3):213–216. doi: 10.1007/BF00373649. [DOI] [PubMed] [Google Scholar]
- Kimoto M., Fathman C. G. Antigen-reactive T cell clones. I. Transcomplementing hybrid I-A-region gene products function effectively in antigen presentation. J Exp Med. 1980 Oct 1;152(4):759–770. doi: 10.1084/jem.152.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnick J. T., Ostberg L., Stegagno M., Kimura A. K., Orn A., Sjöberg O. A rapid method for the separation of functional lymphoid cell populations of human and animal origin on PVP-silica (Percoll) density gradients. Scand J Immunol. 1979;10(6):563–573. doi: 10.1111/j.1365-3083.1979.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Goddeeris B. M., Teale A. J., Groocock C. M., Kemp S. J., Stagg D. A. Cytotoxic T-cells elicited in cattle challenged with Theileria parva (Muguga): evidence for restriction by class I MHC determinants and parasite strain specificity. Parasite Immunol. 1987 Sep;9(5):563–578. doi: 10.1111/j.1365-3024.1987.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Immune response (Ir) genes of the murine major histocompatibility complex. Adv Immunol. 1986;38:31–201. doi: 10.1016/s0065-2776(08)60006-1. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Sigurdardóttir S., Lundén A., Andersson L. Restriction fragment length polymorphism of DQ and DR class II genes of the bovine major histocompatibility complex. Anim Genet. 1988;19(2):133–150. doi: 10.1111/j.1365-2052.1988.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Teale A. J., Baldwin C. L., Ellis J. A., Newson J., Goddeeris B. M., Morrison W. I. Alloreactive bovine T lymphocyte clones: an analysis of function, phenotype, and specificity. J Immunol. 1986 Jun 15;136(12):4392–4398. [PubMed] [Google Scholar]
- Teale A. J., Kemp S. J. A study of BoLA class II antigens with BoT4+ T lymphocyte clones. Anim Genet. 1987;18(1):17–28. doi: 10.1111/j.1365-2052.1987.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Yunis E. J., Matsui Y., Jabara H. H., Geha R. S. HLA-DR-4-associated alloreactivity of an HLA-DR-3-restricted human tetanus toxoid-specific T cell clone: inhibition of both reactivities by an alloantiserum. Eur J Immunol. 1985 Apr;15(4):356–361. doi: 10.1002/eji.1830150410. [DOI] [PubMed] [Google Scholar]
- Usinger W. R., Curie-Cohen M., Benforado K., Pringnitz D., Rowe R., Splitter G. A., Stone W. H. The bovine major histocompatibility complex (BoLa): close linkage of the genes controlling serologically defined antigens and mixed lymphocyte reactivity. Immunogenetics. 1981;14(5):423–428. doi: 10.1007/BF00373322. [DOI] [PubMed] [Google Scholar]
- Watkins D. I., Shadduck J. A., Rudd C. E., Stone M. E., Lewin H. A., Letvin N. L. Isoelectric focusing of bovine major histocompatibility complex class II molecules. Eur J Immunol. 1989 Mar;19(3):567–570. doi: 10.1002/eji.1830190326. [DOI] [PubMed] [Google Scholar]
- van der Poel J. J., Groenen M. A., Dijkhof R. J., Ruyter D., Giphart M. J. The nucleotide sequence of the bovine MHC class II alpha genes: DRA, DOA, and DYA. Immunogenetics. 1990;31(1):29–36. doi: 10.1007/BF00702486. [DOI] [PubMed] [Google Scholar]



