Abstract
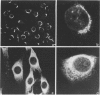
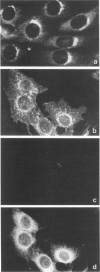
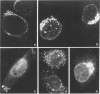
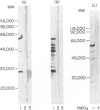
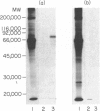
A number of autoreactive monoclonal antibodies have been produced by the fusion of spleen cells from unprimed BALB/c mice. The specificities of two of these monoclonal autoantibodies, MUI 38 and MUI 100, have been further examined. By indirect immunofluorescence, monoclonal antibody MUI 38 showed discrete perinuclear staining of acetone-fixed murine 3T3 fibroblasts, which was similar to that obtained with the Golgi vital stain, C6-NBD-ceramide, and with rhodamine-labelled wheat germ agglutinin. Furthermore, the staining pattern with antibody MUI 38 in cells treated with either monensin, taxol or nocodazol was altered in a manner consistent with the known effects of these drugs on Golgi morphology. In contrast, monoclonal antibody MUI 100 showed a diffuse cytoplasmic staining pattern, similar to FITC-Con A, indicative of reactivity with the endoplasmic reticulum. At high dilutions antibody MUI 100 showed only a perinuclear staining pattern, indicating that MUI 100 reacted with the Golgi as well as the endoplasmic reticulum. Both monoclonal antibodies are IgM kappa class and both showed reactivity with acetone-fixed fibroblasts from a number of species, indicating that the antigens are highly conserved. By immunoblotting with total membranes of murine 3T3 cells under either reducing or non-reducing conditions, monoclonal antibody MUI 100 reacted with a number of components with apparent molecular weights (MW) from 27,000 to 63,000. This reactivity was abolished when the 3T3 membranes were treated with sodium periodate, indicating antibody MUI 100 may be specific for carbohydrate. In addition, MUI 100, but not MUI 38, possessed rheumatoid factor activity, reacting with IgG from normal sera of a number of different species. Furthermore, monoclonal antibody MUI 100 was shown to be specific for the Fc domain of IgG. Absorption of MUI 100 antibody with normal rabbit IgG-Sepharose reduced the anti-endoplasmic reticulum reactivity, therefore both activities are attributable to the same antibody.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Capasso J. M., Abeijon C., Hirschberg C. B. An intrinsic membrane glycoprotein of the golgi apparatus with O-linked N-acetylglucosamine facing the cytosol. J Biol Chem. 1988 Dec 25;263(36):19778–19782. [PubMed] [Google Scholar]
- Conger J. D., Pike B. L., Nossal G. J. Clonal analysis of the anti-DNA repertoire of murine B lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2931–2935. doi: 10.1073/pnas.84.9.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote R. J., Morrissey D. M., Houghton A. N., Beattie E. J., Jr, Oettgen H. F., Old L. J. Generation of human monoclonal antibodies reactive with cellular antigens. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2026–2030. doi: 10.1073/pnas.80.7.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Dresser D. W. Most IgM-producing cells in the mouse secrete auto-antibodies (rheumatoid factor). Nature. 1978 Aug 3;274(5670):480–483. doi: 10.1038/274480a0. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brands R., Rothman J. E. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Preferential induction of autoantibody secretion in polyclonal activation by peptidoglycan and lipopolysaccharide. I. In vitro studies. J Immunol. 1982 Mar;128(3):1018–1025. [PubMed] [Google Scholar]
- Fritzler M. J., Etherington J., Sokoluk C., Kinsella T. D., Valencia D. W. Antibodies from patients with autoimmune disease react with a cytoplasmic antigen in the Golgi apparatus. J Immunol. 1984 Jun;132(6):2904–2908. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn I., Gleeson P. A., Toh B. H. Gastric parietal cell antigens of 60-90, 92, and 100-120 kDa associated with autoimmune gastritis and pernicious anemia. Role of N-glycans in the structure and antigenicity of the 60-90-kDa component. J Biol Chem. 1989 Nov 5;264(31):18768–18774. [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Harms E., Kern H., Schneider J. A. Human lysosomes can be purified from diploid skin fibroblasts by free-flow electrophoresis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6139–6143. doi: 10.1073/pnas.77.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. W., Haltiwanger R. S., Holt G. D., Kelly W. G. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem. 1989;58:841–874. doi: 10.1146/annurev.bi.58.070189.004205. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. A vital stain for the Golgi apparatus. Science. 1985 May 10;228(4700):745–747. doi: 10.1126/science.2581316. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J. Clonal analysis of autoantibody-producing cell precursors in the preimmune B cell repertoire. J Immunol. 1988 Dec 15;141(12):4118–4123. [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Prabhakar B. S., Saegusa J., Onodera T., Notkins A. L. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984 Dec;133(6):2815–2817. [PubMed] [Google Scholar]
- Ralton J. E., Jackson H. J., Zanoni M., Gleeson P. A. Effect of glycosylation inhibitors on the structure and function of the murine transferrin receptor. Eur J Biochem. 1989 Dec 22;186(3):637–647. doi: 10.1111/j.1432-1033.1989.tb15254.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez J. L., Gelpi C., Thomson T. M., Real F. J., Fernández J. Anti-golgi complex autoantibodies in a patient with Sjögren syndrome and lymphoma. Clin Exp Immunol. 1982 Sep;49(3):579–586. [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tassin A. M., Paintrand M., Berger E. G., Bornens M. The Golgi apparatus remains associated with microtubule organizing centers during myogenesis. J Cell Biol. 1985 Aug;101(2):630–638. doi: 10.1083/jcb.101.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Murine natural monoclonal autoantibodies: a study of their polyspecificities and their affinities. Immunol Rev. 1986 Dec;94:99–112. doi: 10.1111/j.1600-065x.1986.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J. R., Pedersen J. S., Chalmers P. J., Toh B. H. Hybrids from normal, germ free, nude and neonatal mice produce monoclonal autoantibodies to eight different intracellular structures. Clin Exp Immunol. 1985 May;60(2):417–426. [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Henkart M., Klausner R., Sandoval I. V. Role of microtubules in the distribution of the Golgi apparatus: effect of taxol and microinjected anti-alpha-tubulin antibodies. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4286–4290. doi: 10.1073/pnas.80.14.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]