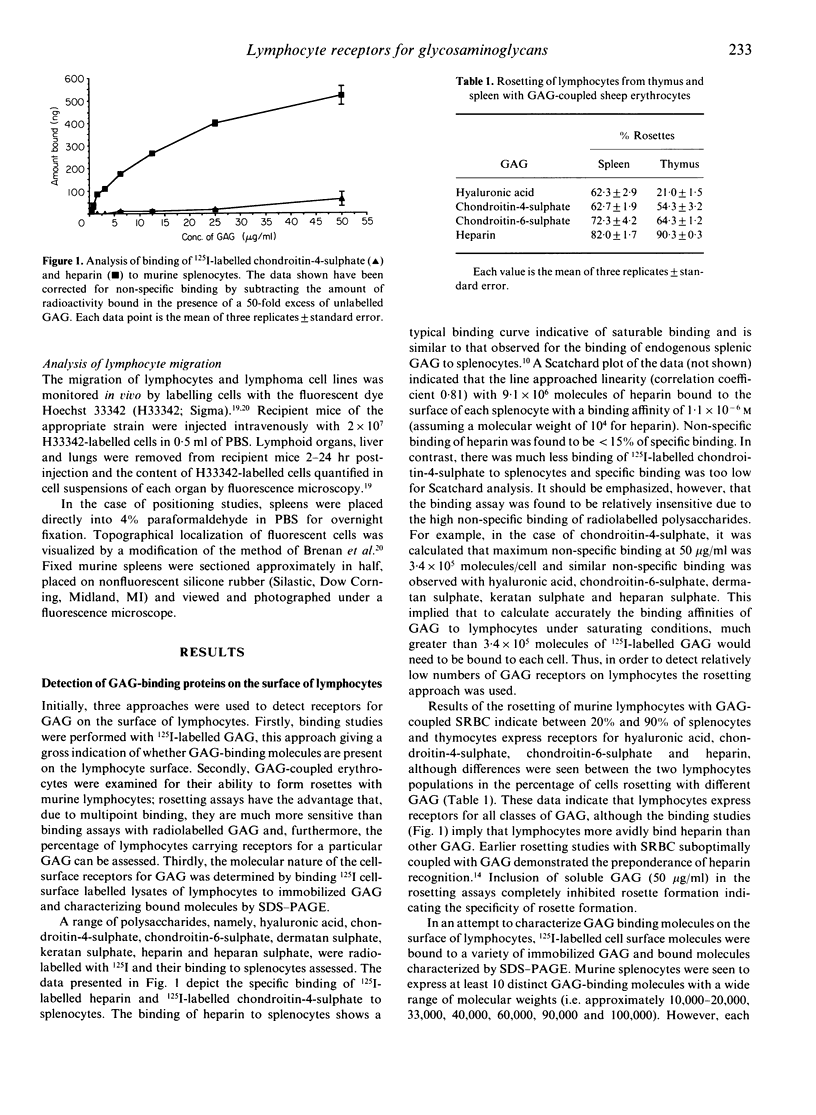
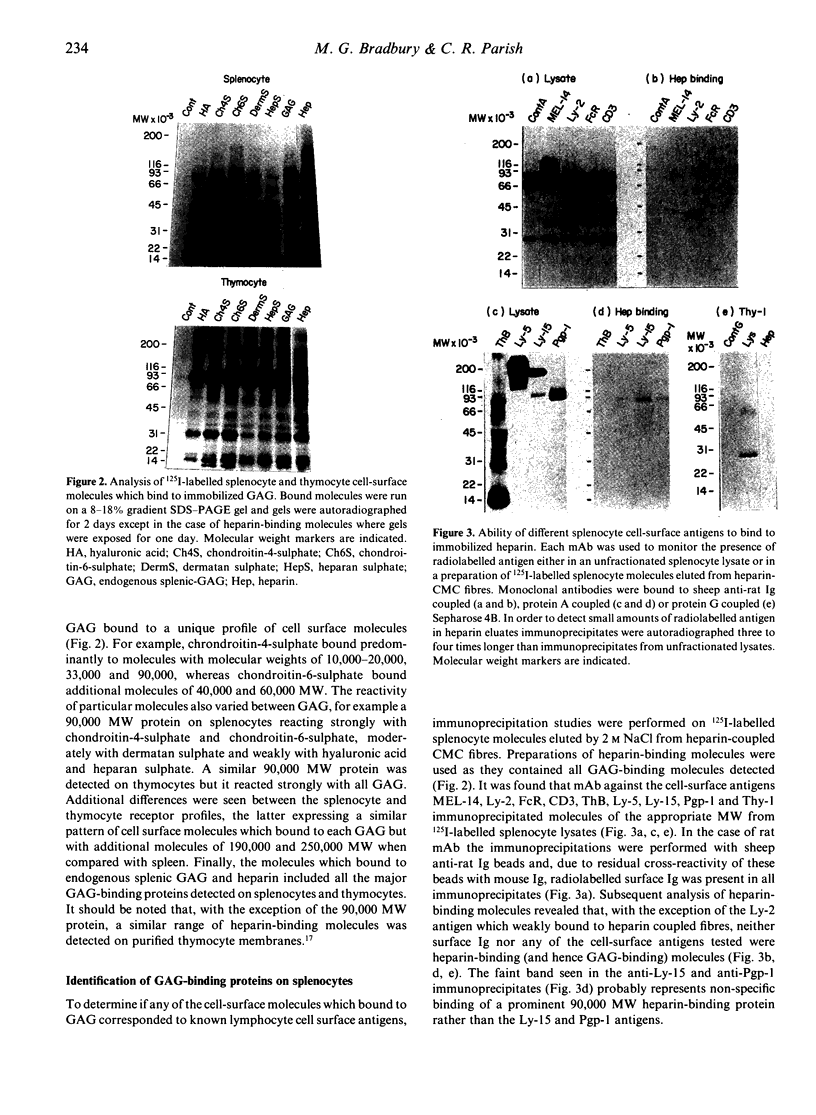
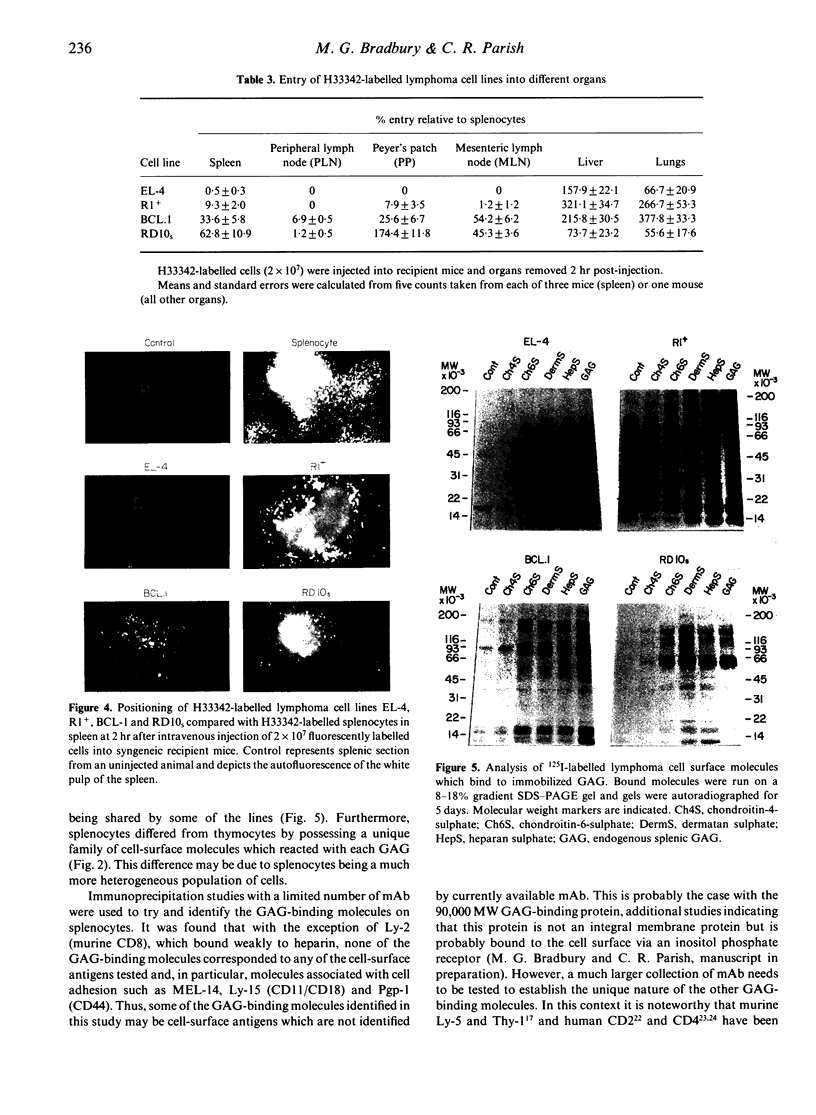
Abstract
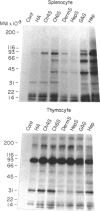
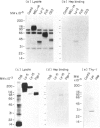
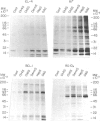
This paper describes attempts to isolate and characterize glycosaminoglycan (GAG)-binding molecules on the surface of lymphocytes and lymphoma cell lines and relate their expression to splenic and lymph node homing capacity. Initial binding studies with radiolabelled GAG and rosetting studies with GAG-coupled erythrocytes revealed that there are receptors on lymphocytes for the major classes of GAG (i.e. hyaluronic acid, chrondroitin sulfates, heparin), but lymphocytes bind heparin much more avidly than other GAG species. Analysis of the binding of solubilized radiolabelled cell-surface molecules to immobilized GAG revealed cell-type specific expression of GAG-binding molecules. Thus, each of four lymphoma cell lines tested gave a characteristic pattern of GAG-binding molecules, some molecules being unique to a particular cell line and others being shared by some of the lines. Similarly, splenocytes expressed at least 10 distinct GAG-binding molecules with molecular weights (MW) ranging from 10,000 to 100,000, whereas thymocytes expressed additional GAG-binding proteins of 190,000 and 250,000 MW. Furthermore, splenocytes differed from thymocytes by possessing a unique family of cell-surface molecules which reacted with each GAG. Immunoprecipitation studies demonstrated that the GAG-binding molecules on splenocytes did not correspond to any of the cell-surface antigens tested, notably the cell adhesion molecules MEL-14, CD11/CD18 and CD44, although CD8 bound weakly to heparin. Four lymphoma cell lines with well-characterized migration properties were examined for GAG-binding molecules which may control lymphocyte migration. It was found that no one GAG-binding protein could be correlated with the entry of cells into a particular lymphoid organ. Nevertheless, the role of GAG-binding molecules in the subsequent positioning of lymphocytes within lymphoid organs requires further investigation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury M. G., Parish C. R. Receptors on lymphocytes for endogenous splenic glycosaminoglycans. Immunology. 1989 Apr;66(4):546–553. [PMC free article] [PubMed] [Google Scholar]
- Bradfield J. W., Born G. V. Lymphocytosis produced by heparin and other sulphated polysaccharides in mice and rats. Cell Immunol. 1974 Oct;14(1):22–32. doi: 10.1016/0008-8749(74)90165-8. [DOI] [PubMed] [Google Scholar]
- Brenan M., Parish C. R. Intracellular fluorescent labelling of cells for analysis of lymphocyte migration. J Immunol Methods. 1984 Nov 16;74(1):31–38. doi: 10.1016/0022-1759(84)90364-8. [DOI] [PubMed] [Google Scholar]
- Brenan M., Parish C. R. Modification of lymphocyte migration by sulfated polysaccharides. Eur J Immunol. 1986 Apr;16(4):423–430. doi: 10.1002/eji.1830160419. [DOI] [PubMed] [Google Scholar]
- Brenan M., Parish C. R., Schoefl G. I. Topographical studies of lymphocyte localization using an intracellular fluorochrome. Anat Rec. 1985 Nov;213(3):421–428. doi: 10.1002/ar.1092130308. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980 Jul;10(7):556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Glabe C. G., Harty P. K., Rosen S. D. Preparation and properties of fluorescent polysaccharides. Anal Biochem. 1983 Apr 15;130(2):287–294. doi: 10.1016/0003-2697(83)90590-0. [DOI] [PubMed] [Google Scholar]
- JANSEN C. R., CRONKITE E. P., MATHER G. C., NIELSEN N. O., RAI K., ADAMIK E. R., SIPE C. R. Studies on lymphocytes. II. The production of lymphocytosis by intravenous heparin in calves. Blood. 1962 Oct;20:443–452. [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Yednock T. A., Dowbenko D., Fennie C., Rodriguez H., Nguyen T., Stachel S., Rosen S. D. Cloning of a lymphocyte homing receptor reveals a lectin domain. Cell. 1989 Mar 24;56(6):1045–1055. doi: 10.1016/0092-8674(89)90637-5. [DOI] [PubMed] [Google Scholar]
- Lederman S., Gulick R., Chess L. Dextran sulfate and heparin interact with CD4 molecules to inhibit the binding of coat protein (gp120) of HIV. J Immunol. 1989 Aug 15;143(4):1149–1154. [PubMed] [Google Scholar]
- McCarthy K. J., Accavitti M. A., Couchman J. R. Immunological characterization of a basement membrane-specific chondroitin sulfate proteoglycan. J Cell Biol. 1989 Dec;109(6 Pt 1):3187–3198. doi: 10.1083/jcb.109.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R., Hogarth P. M., McKenzie I. F. Evidence that Thy-1 and Ly-5 (T-200) antigens interact with sulphated carbohydrates. Immunol Cell Biol. 1988 Jan;66(Pt 3):221–230. doi: 10.1038/icb.1988.28. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Low L., Warren H. S., Cunningham A. L. A polyanion binding site on the CD4 molecule. Proximity to the HIV-gp120 binding region. J Immunol. 1990 Aug 15;145(4):1188–1195. [PubMed] [Google Scholar]
- Parish C. R., McPhun V., Warren H. S. Is a natural ligand of the T lymphocyte CD2 molecule a sulfated carbohydrate? J Immunol. 1988 Nov 15;141(10):3498–3504. [PubMed] [Google Scholar]
- Sasaki S., Suchi T. Mobilization of lymphocytes from lymph nodes and spleen by polysaccharide polysulphate. Nature. 1967 Dec 9;216(5119):1013–1014. doi: 10.1038/2161013a0. [DOI] [PubMed] [Google Scholar]
- Siegelman M. H., van de Rijn M., Weissman I. L. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989 Mar 3;243(4895):1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- Slavin S., Strober S. Spontaneous murine B-cell leukaemia. Nature. 1978 Apr 13;272(5654):624–626. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Butcher E. C., Stoolman L. M., Rosen S. D. Receptors involved in lymphocyte homing: relationship between a carbohydrate-binding receptor and the MEL-14 antigen. J Cell Biol. 1987 Mar;104(3):725–731. doi: 10.1083/jcb.104.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock T. A., Rosen S. D. Lymphocyte homing. Adv Immunol. 1989;44:313–378. doi: 10.1016/s0065-2776(08)60645-8. [DOI] [PubMed] [Google Scholar]