Abstract
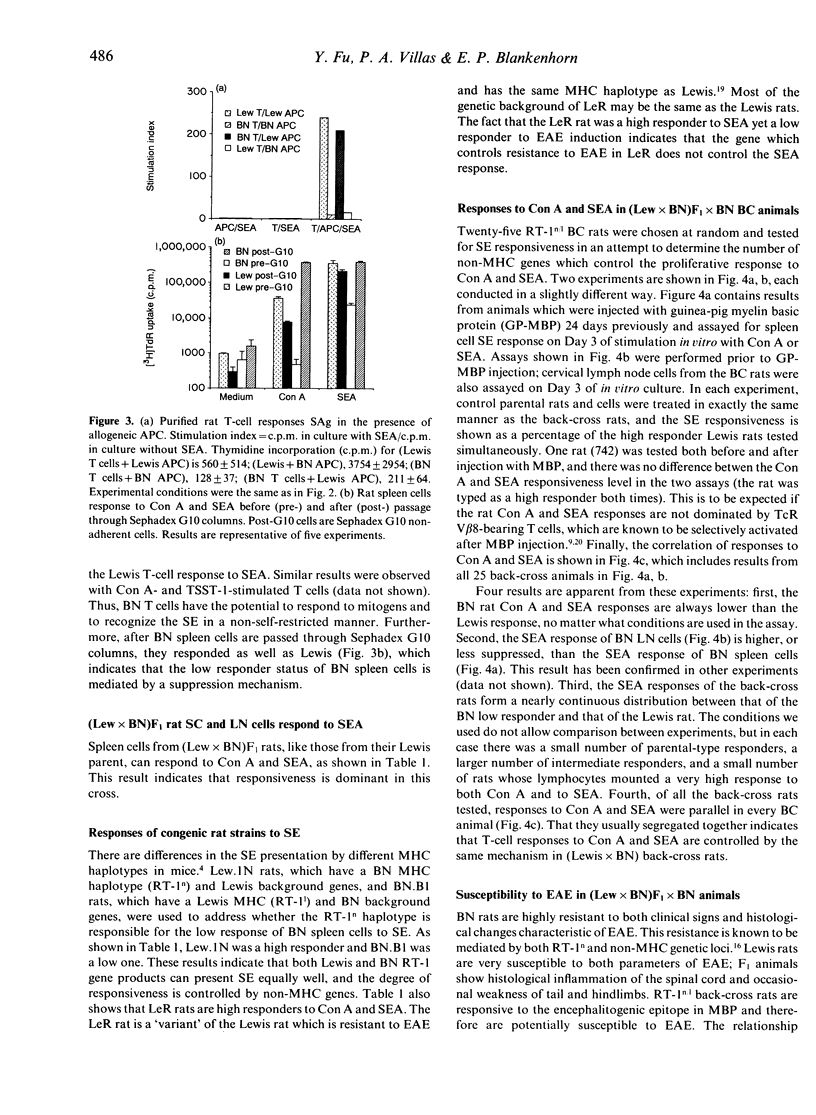
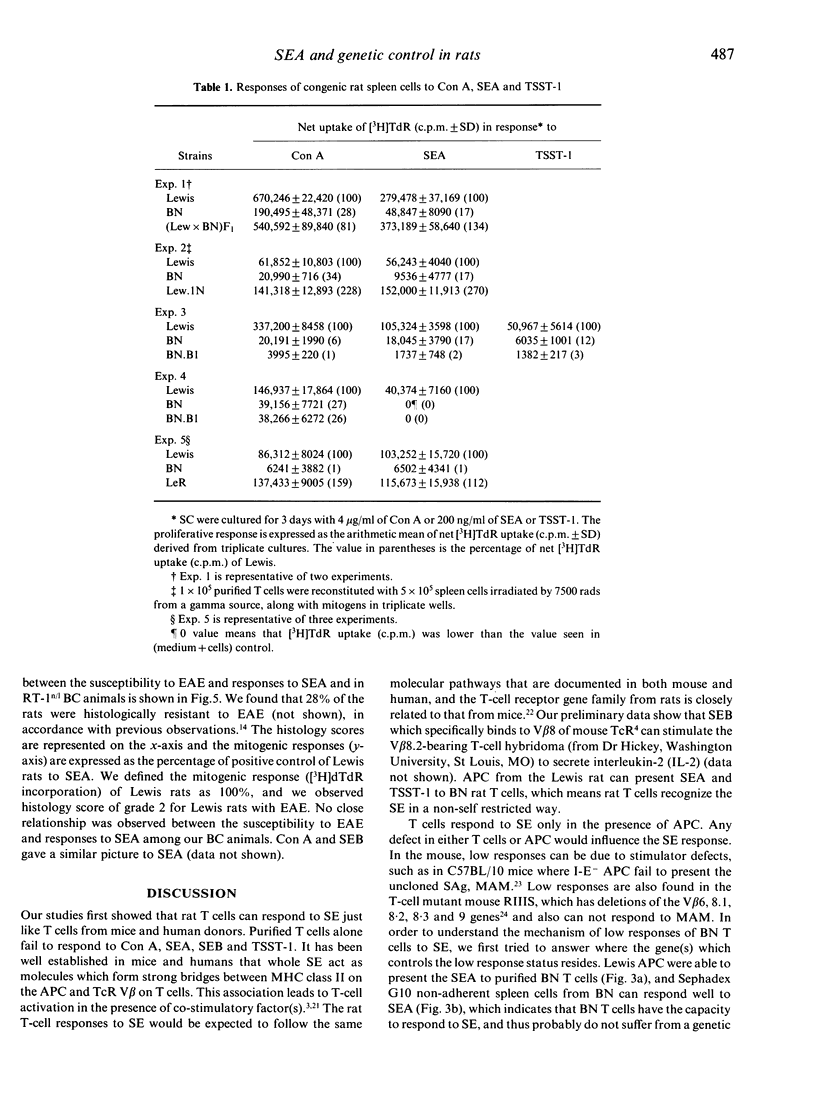
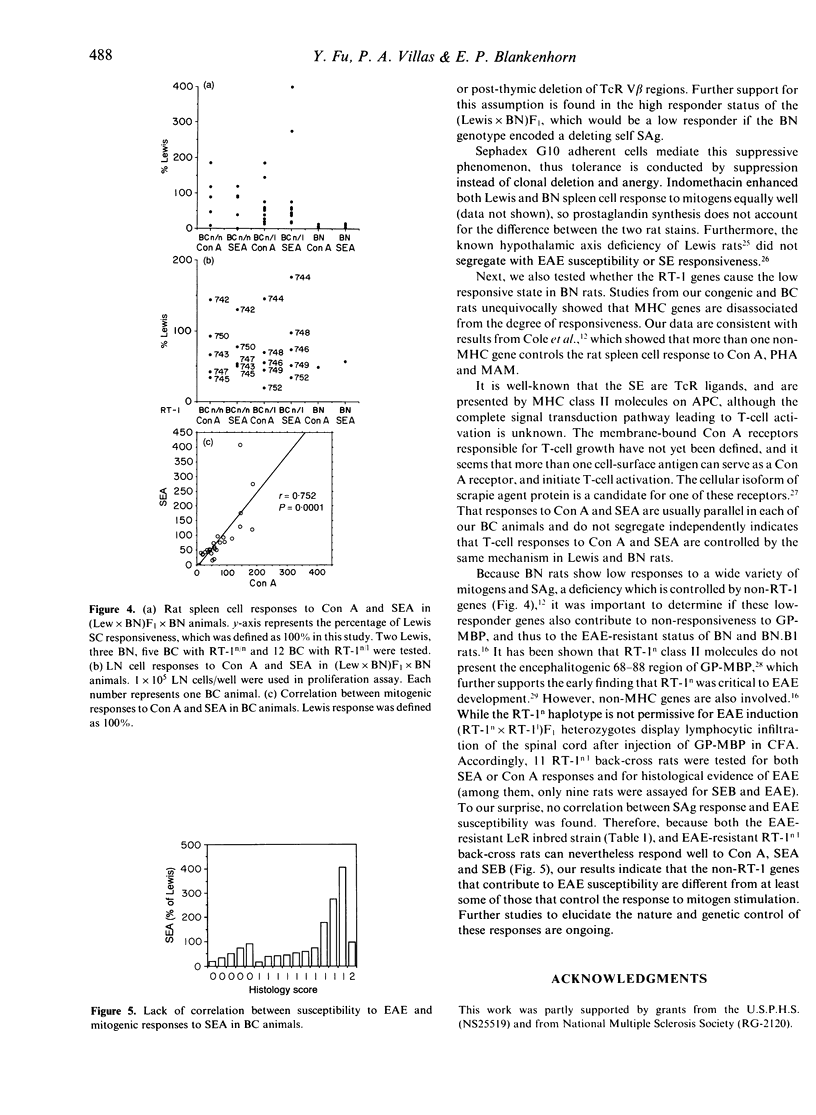
Rat T cells, like those of mouse and human origin, respond strongly to superantigens (SAg) derived from Staphylococcus aureus enterotoxins A and B (SEA, SEB). Lewis and ACI are high responders, whereas Brown Norway (BN) is a low responder. Congenic and back-cross rat studies indicate that the degree of responsiveness is controlled by at least one non-MHC gene. The action of these genes may reside in the antigen-presenting cells (APC), since both Sephadex G10 non-adherent BN spleen cells and purified BN T cells in the presence of Lewis APC can respond well to SE. Responses to concanavalin A (Con A) and SEA generally segregate together in back-cross rats. Surprisingly, the degree of responsiveness to Con A and SEA is not correlated with the susceptibility to experimental allergic encephalomyelitis (EAE) either in independently derived inbred rat strains or in (Lewis x BN) x BN back-cross rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashman N. R., Loertscher R., Nalbantoglu J., Shaw I., Kascsak R. J., Bolton D. C., Bendheim P. E. Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell. 1990 Apr 6;61(1):185–192. doi: 10.1016/0092-8674(90)90225-4. [DOI] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. III. Ir gene control of lymphocyte transformation correlates with binding of the mitogen to specific Ia-bearing cells. J Immunol. 1982 Oct;129(4):1352–1359. [PubMed] [Google Scholar]
- Cole B. C., Griffiths M. M., Sullivan G. J., Ward J. R. Role of non-RT1 genes in the response of rat lymphocytes to Mycoplasma arthritidis T cell mitogen, concanavalin A and phytohemagglutinin. J Immunol. 1986 Apr 1;136(7):2364–2369. [PubMed] [Google Scholar]
- Cole B. C., Kartchner D. R., Wells D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis (MAM). VIII. Selective activation of T cells expressing distinct V beta T cell receptors from various strains of mice by the "superantigen" MAM. J Immunol. 1990 Jan 15;144(2):425–431. [PubMed] [Google Scholar]
- Cole B. C., Kartchner D. R., Wells D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. VII. Responsiveness is associated with expression of a product(s) of the V beta 8 gene family present on the T cell receptor alpha/beta for antigen. J Immunol. 1989 Jun 15;142(12):4131–4137. [PubMed] [Google Scholar]
- Davis B. K., Kunz H. W., Shonnard J. W., Gill T. J., 3rd Immune response to poly(Glu52Lys33Tyr15) in congenic rats. Transplant Proc. 1981 Jun;13(2):1378–1382. [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Gasser D. L., Newlin C. M., Palm J., Gonatas N. K. Genetic control of susceptibility to experimental allergic encephalomyelitis in rats. Science. 1973 Aug 31;181(4102):872–873. doi: 10.1126/science.181.4102.872. [DOI] [PubMed] [Google Scholar]
- Happ M. P., Wettstein P., Dietzschold B., Heber-Katz E. Genetic control of the development of experimental allergic encephalomyelitis in rats. Separation of MHC and non-MHC gene effects. J Immunol. 1988 Sep 1;141(5):1489–1494. [PubMed] [Google Scholar]
- Heber-Katz E., Acha-Orbea H. The V-region disease hypothesis: evidence from autoimmune encephalomyelitis. Immunol Today. 1989 May;10(5):164–169. doi: 10.1016/0167-5699(89)90174-6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Marrack P., Blackman M., Kushnir E., Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990 Feb 1;171(2):455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- McKearn T. J., Fitch F. W., Smilek D. E., Sarmiento M., Stuart F. P. Properties of rat anti-MHC antibodies produced by cloned rat-mouse hybridomas. Immunol Rev. 1979;47:91–115. doi: 10.1111/j.1600-065x.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Newlin C. M., Gasser D. L. Genetic control of the in vitro responses of rat peripheral blood lymphocytes to phytohemagglutinin and concanavalin A. J Immunol. 1973 Mar;110(3):622–628. [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J Infect Dis. 1985 Jan;151(1):65–72. doi: 10.1093/infdis/151.1.65. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Kappler J. W., Marrack P. Tolerance to self antigens shapes the T-cell repertoire. Immunol Rev. 1989 Feb;107:125–139. doi: 10.1111/j.1600-065x.1989.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Singer D. E., Moore M. J., Williams R. M. EAE in rat bone marrow chimeras: analysis of the cellular mechanism of BN resistance. J Immunol. 1981 Apr;126(4):1553–1557. [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman F. J., Perryman L. E., Hinrichs D. J., Coe J. E. Genetic resistance to the induction of experimental allergic encephalomyelitis in Lewis rats. I. Genetic analysis of an apparent mutant strain with phenotypic resistance to experimental allergic encephalomyelitis. J Exp Med. 1981 Jan 1;153(1):61–74. doi: 10.1084/jem.153.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Williams R. M., Moore M. J. Linkage of susceptibility to experimental allergic encephalomyelitis to the major histocompatibility locus in the rat. J Exp Med. 1973 Oct 1;138(4):775–783. doi: 10.1084/jem.138.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]


