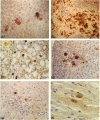
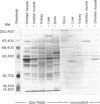
Abstract
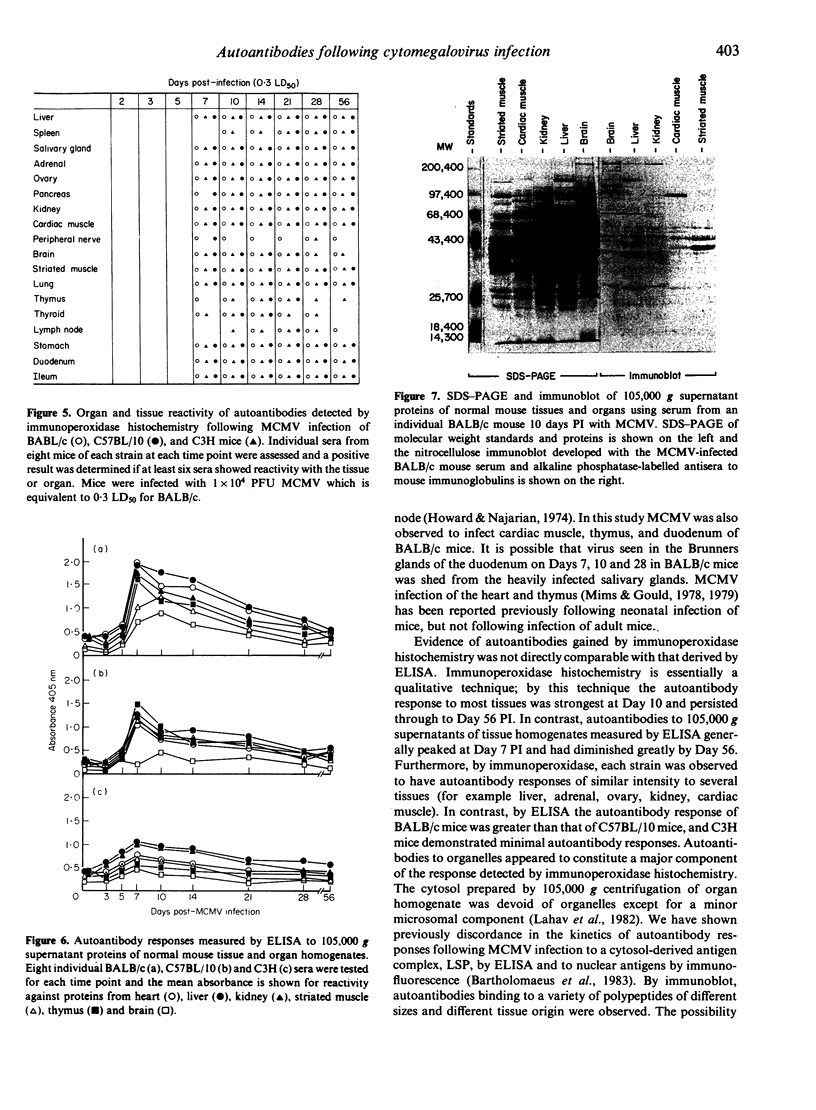
Multiple autoantibodies were found in the sera of BALB/c, C57BL/10 and C3H mice following mouse cytomegalovirus (MCMV) infection. The complex pattern of intra-organ, intratissue and intracellular reactivity observed by immunoperoxidase histochemistry suggested that many autoantibodies of varying specificities were elicited. This evidence from immunoperoxidase histochemistry was confirmed by immunoblot, where autoantibodies binding to polypeptides of a variety of sizes and tissues of origin were observed. In addition to these central findings, the tissue and organ distribution of MCMV in three mouse strains of differing genetic resistance were described. No correlation was found between the distribution of virus and the tissue and organ specificity of the autoantibodies produced following infection. Inflammatory responses accompanied MCMV infection in a number of tissues. In BALB/c mice, myocarditis and salivary gland inflammation were evident at Day 56 post-infection in the absence of MCMV, but in the presence of autoantibodies to cardiac muscle and salivary duct epithelium. This model for virus-induced autoimmunity can be applied to studies of the relationship between virus infection, autoimmunity and disease.
Full text
PDF

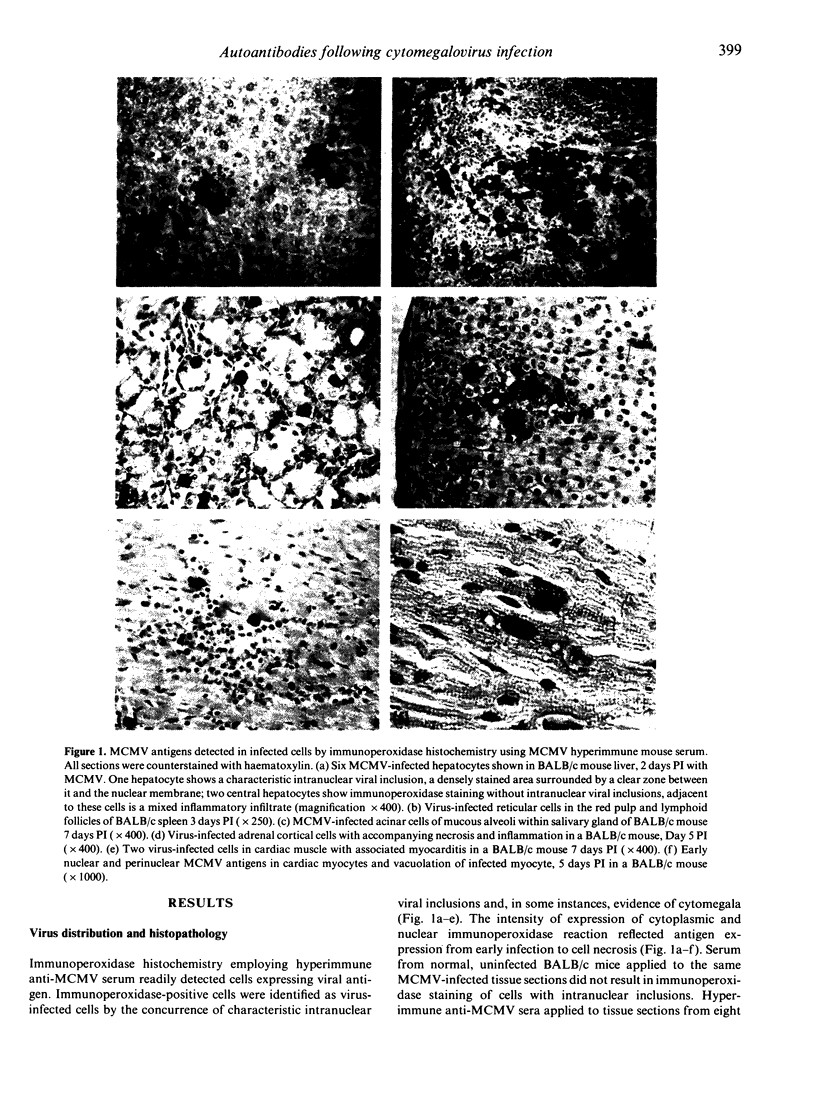
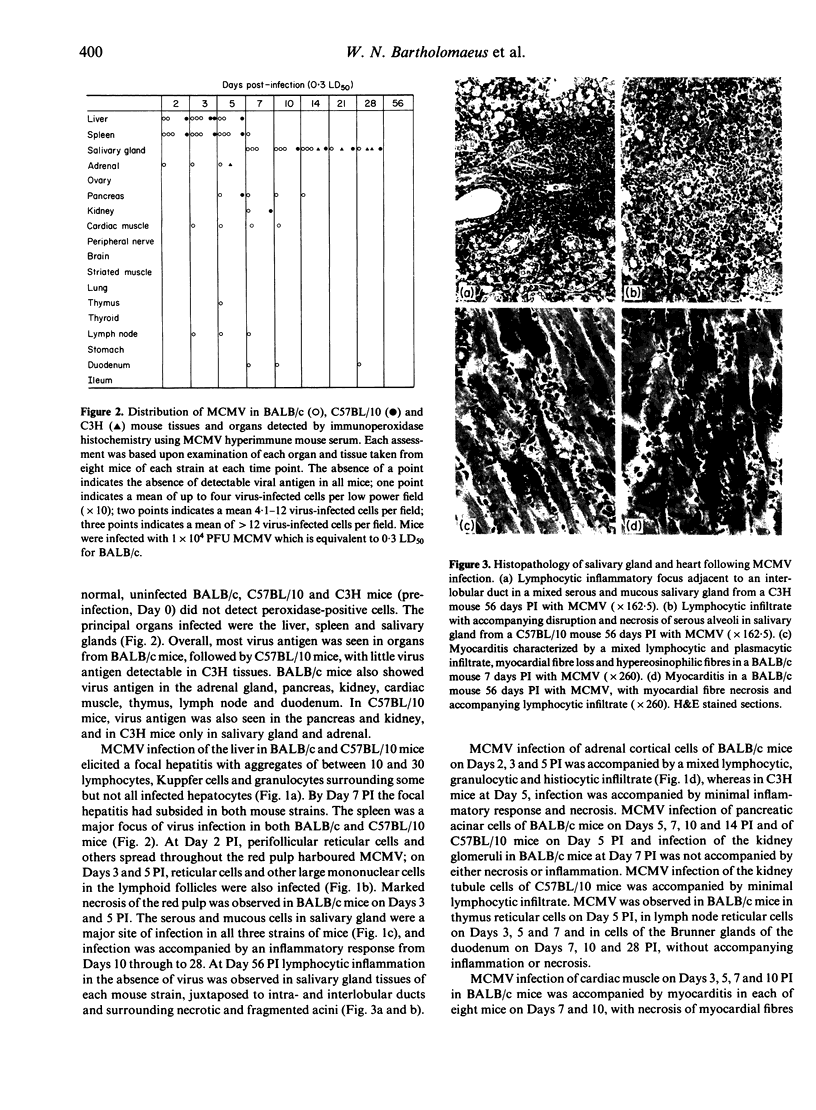




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. E., Shellam G. R. Genetic control of murine cytomegalovirus infection: virus titres in resistant and susceptible strains of mice. Arch Virol. 1984;81(1-2):139–150. doi: 10.1007/BF01309303. [DOI] [PubMed] [Google Scholar]
- Andersen P., Andersen H. K. Smooth-muscle antibodies and other tissue antibodies in cytomegalovirus infection. Clin Exp Immunol. 1975 Oct;22(1):22–29. [PMC free article] [PubMed] [Google Scholar]
- Bagchi S., Das K. M. Detection and partial characterization of Crohn's disease tissue specific proteins recognized by Crohn's disease sera. Clin Exp Immunol. 1984 Jan;55(1):41–48. [PMC free article] [PubMed] [Google Scholar]
- Bartholomaeus W. N., Shellam G. R., Allan J. E., Reed W. D., Joske R. A. Autoantibodies to liver-specific lipoprotein following hepatitis induced by mouse cytomegalovirus. Clin Exp Immunol. 1983 Apr;52(1):89–97. [PMC free article] [PubMed] [Google Scholar]
- Bartholomaeus W. N., Swanson N. R., Reed W. D., O'Donoghue H. L., Foti D., Papadimitriou J. M. The relationship between liver-specific lipoprotein and the hepatocyte plasma membrane. Immunology. 1987 Mar;60(3):321–329. [PMC free article] [PubMed] [Google Scholar]
- Chalmer J. E., Mackenzie J. S., Stanley N. F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977 Oct;37(1):107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- Cooke A., Lydyard P. M., Roitt I. M. Autoimmunity and idiotypes. Lancet. 1984 Sep 29;2(8405):723–725. doi: 10.1016/s0140-6736(84)92628-x. [DOI] [PubMed] [Google Scholar]
- Dao M. L. An improved method of antigen detection on nitrocellulose: in situ staining of alkaline phosphatase conjugated antibody. J Immunol Methods. 1985 Oct 10;82(2):225–231. doi: 10.1016/0022-1759(85)90354-0. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Mackenzie J. S., Stanley N. F. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect Immun. 1981 Apr;32(1):277–286. doi: 10.1128/iai.32.1.277-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., Horita M., Suzuki H., Notkins A. L. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science. 1983 Apr 15;220(4594):304–306. doi: 10.1126/science.6301002. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Najarian J. S. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974 Sep;18(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. L., Goldberg L. S., Johnson B. L., Jr, Derechin M. M., Barnett E. V. Immunologic abnormalities induced by postperfusion cytomegalovirus infection. Ann Intern Med. 1970 Oct;73(4):553–558. doi: 10.7326/0003-4819-73-4-553. [DOI] [PubMed] [Google Scholar]
- Lahav M., Schoenfeld N., Epstein O., Atsmon A. A method for obtaining high recovery of purified subcellular fractions of rat liver homogenate. Anal Biochem. 1982 Mar 15;121(1):114–122. doi: 10.1016/0003-2697(82)90563-2. [DOI] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Gould J. Infection of salivary glands, kidneys, adrenals, ovaries and epithelia by murine cytomegalovirus. J Med Microbiol. 1979 Feb;12(1):113–122. doi: 10.1099/00222615-12-1-113. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Gould J. The role of macrophages in mice infected with murine cytomegalovirus. J Gen Virol. 1978 Oct;41(1):143–153. doi: 10.1099/0022-1317-41-1-143. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Eddleston A. L. Mechanisms of liver cell injury in acute and chronic hepatitis B. Semin Liver Dis. 1984 Feb;4(1):47–58. doi: 10.1055/s-2008-1040645. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Kingsbury D. T., Oldstone M. B. Pathogenesis of cytomegalovirus infection. Distribution of viral products, immune complexes and autoimmunity during latent murine infection. J Gen Virol. 1976 Nov;33(2):267–280. doi: 10.1099/0022-1317-33-2-267. [DOI] [PubMed] [Google Scholar]
- Onodera T., Toniolo A., Ray U. R., Jenson A. B., Knazek R. A., Notkins A. L. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J Exp Med. 1981 Jun 1;153(6):1457–1473. doi: 10.1084/jem.153.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar B. S., Saegusa J., Onodera T., Notkins A. L. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984 Dec;133(6):2815–2817. [PubMed] [Google Scholar]
- Shillitoe E. J., Daniels T. E., Whitcher J. P., Vibeke Strand C., Talal N., Greenspan J. S. Antibody to cytomegalovirus in patients with Sjögren's syndrome. As determined by an enzyme-linked immunosorbent assay. Arthritis Rheum. 1982 Mar;25(3):260–265. doi: 10.1002/art.1780250303. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Steinberg A. D. Autoimmunity--a perspective. Annu Rev Immunol. 1983;1:175–210. doi: 10.1146/annurev.iy.01.040183.001135. [DOI] [PubMed] [Google Scholar]
- Swanson N. R., Bartholomaeus W. N., Reed W. D., Joske R. A. An enzyme-linked immunosorbent assay for the detection of hepatocyte plasma membrane antibodies. J Immunol Methods. 1985 Dec 17;85(1):203–216. doi: 10.1016/0022-1759(85)90288-1. [DOI] [PubMed] [Google Scholar]
- Taylor P. V., Scott J. S., Gerlis L. M., Esscher E., Scott O. Maternal antibodies against fetal cardiac antigens in congenital complete heart block. N Engl J Med. 1986 Sep 11;315(11):667–672. doi: 10.1056/NEJM198609113151103. [DOI] [PubMed] [Google Scholar]
- Tinghitella T. J., Booss J. Enhanced immune response late in primary cytomegalovirus infection of mice. J Immunol. 1979 Jun;122(6):2442–2446. [PubMed] [Google Scholar]
- Wager O., Räsänen J. A., Hagman A., Klemola E. Mixed cryoimmunoglobulinaemia in infectious mononucleois and Cytomegalovirus mononucleosis. Int Arch Allergy Appl Immunol. 1968;34(4):345–361. doi: 10.1159/000230129. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Wege H., ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983 Sep 8;305(5930):150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Hauser S. L. Neuroimmunology I: Immunoregulation in neurological disease. Ann Neurol. 1982 May;11(5):437–449. doi: 10.1002/ana.410110502. [DOI] [PubMed] [Google Scholar]