Abstract
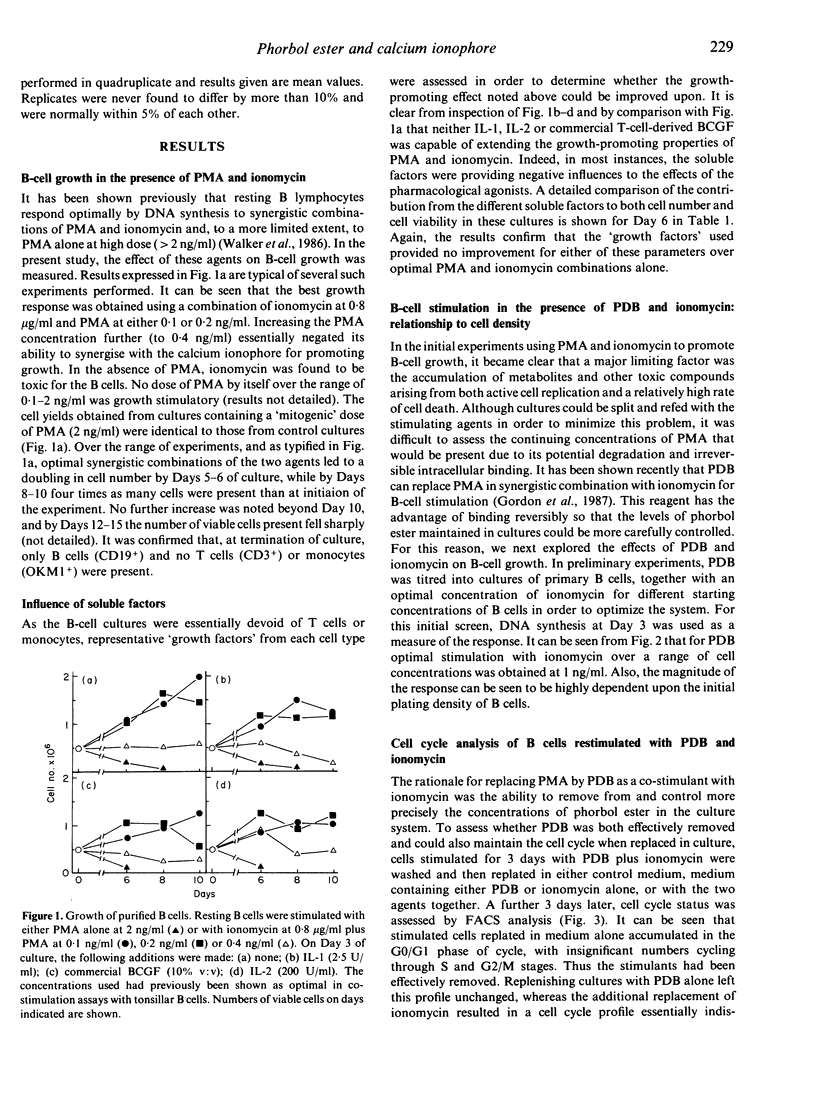
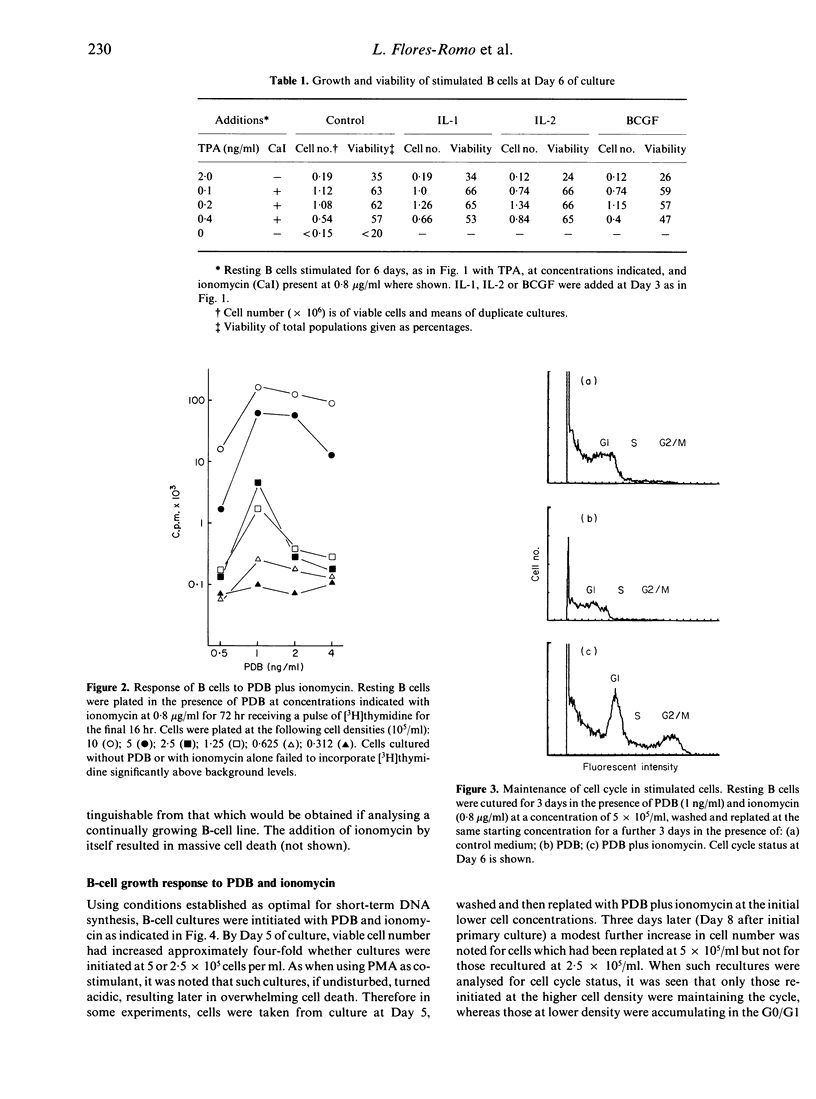
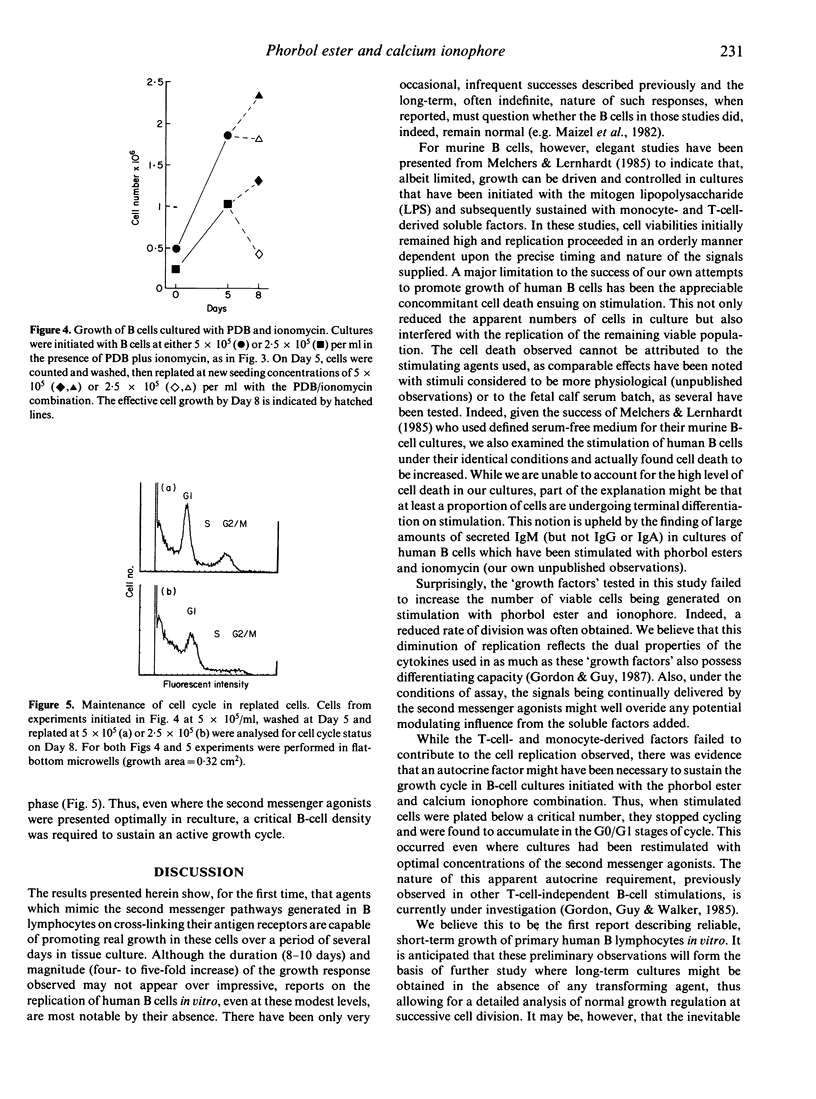
Highly purified, resting B cells could be induced to grow for up to 10 days by culturing in the presence of a synergistic combination of a tumour-promoting phorbol ester and the calcium ionophore ionomycin. In spite of evident cell death occurring, four to five times as many viable B lymphocytes could be harvested at the end of culture than were initially plated. Soluble factors derived from T cells (interleukin-2, commercial B-cell growth factor) or monocytes (interleukin-1) failed to augment further the growth-promotion observed. Evidence is presented to suggest that an autocrine component might be necessary for maintenance of the cell-cycle and growth initiated by the phorbol ester and calcium ionophore combination. The significance of these findings to B-cell physiology are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon J., Guy G., Walker L. Autocrine models of B-lymphocyte growth. I. Role of cell contact and soluble factors in T-independent B-cell responses. Immunology. 1985 Oct;56(2):329–335. [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Millsum M. J., Guy G. R., Ledbetter J. A. Synergistic interaction between interleukin 4 and anti-Bp50 (CDw40) revealed in a novel B cell restimulation assay. Eur J Immunol. 1987 Oct;17(10):1535–1538. doi: 10.1002/eji.1830171026. [DOI] [PubMed] [Google Scholar]
- Guy G. R., Bunce C. M., Gordon J., Michell R. H., Brown G. A combination of calcium ionophore and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) stimulates the growth of purified resting B cells. Scand J Immunol. 1985 Nov;22(5):591–596. doi: 10.1111/j.1365-3083.1985.tb01919.x. [DOI] [PubMed] [Google Scholar]
- Maizel A., Sahasrabuddhe C., Mehta S., Morgan J., Lachman L., Ford R. Biochemical separation of a human B cell mitogenic factor. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5998–6002. doi: 10.1073/pnas.79.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed M. D., Gordon J., Ley S. J., Edgar D., Hughes-Jones N. C. Senescence of a human lymphoblastoid clone producing anti-Rhesus(D). Eur J Immunol. 1985 Jul;15(7):742–746. doi: 10.1002/eji.1830150720. [DOI] [PubMed] [Google Scholar]
- Melchers F., Lernhardt W. Three restriction points in the cell cycle of activated murine B lymphocytes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7681–7685. doi: 10.1073/pnas.82.22.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Walker L., Guy G., Brown G., Rowe M., Milner A. E., Gordon J. Control of human B-lymphocyte replication. I. Characterization of novel activation states that precede the entry of G0 B cells into cycle. Immunology. 1986 Aug;58(4):583–589. [PMC free article] [PubMed] [Google Scholar]


