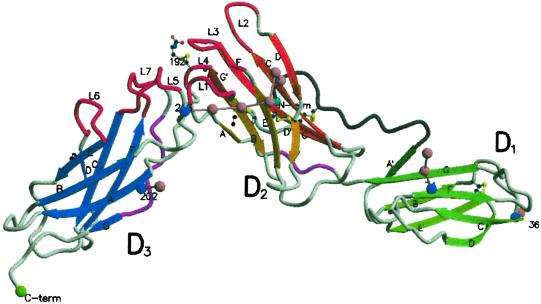
Fig 1.
A molscript (56, 57) diagram of sIL-6R indicating the β-sheet arrangement (shades of green, orange, and blue for domains D1, D2, and D3, respectively). The different β-strand shades represent the separate β-sheets in the structure. Four asparagine-linked sites (blue spheres) are shown with their associated carbohydrate moieties represented by pink spheres linked by pink bonds. The N-terminal residue 1–15 (gray tube) is tethered to strand F of D2 by a disulfide bond. The 310 helices (purple) and the loops (red) L1–L7 [L1–L7 consist of the polypeptides S106–N110 (L1), K133–P138 (L2), A160–F168 (L3), Q190–G193 (L4), S227–R233 (L5), M250–H256 (L6) and Q276–Q281 (L7)] that could interact with IL-6 around the juncture of D2 and D3 are shown. The single cysteine residue disulfide linked to C192 is also shown, as are the disulfide links.