Abstract
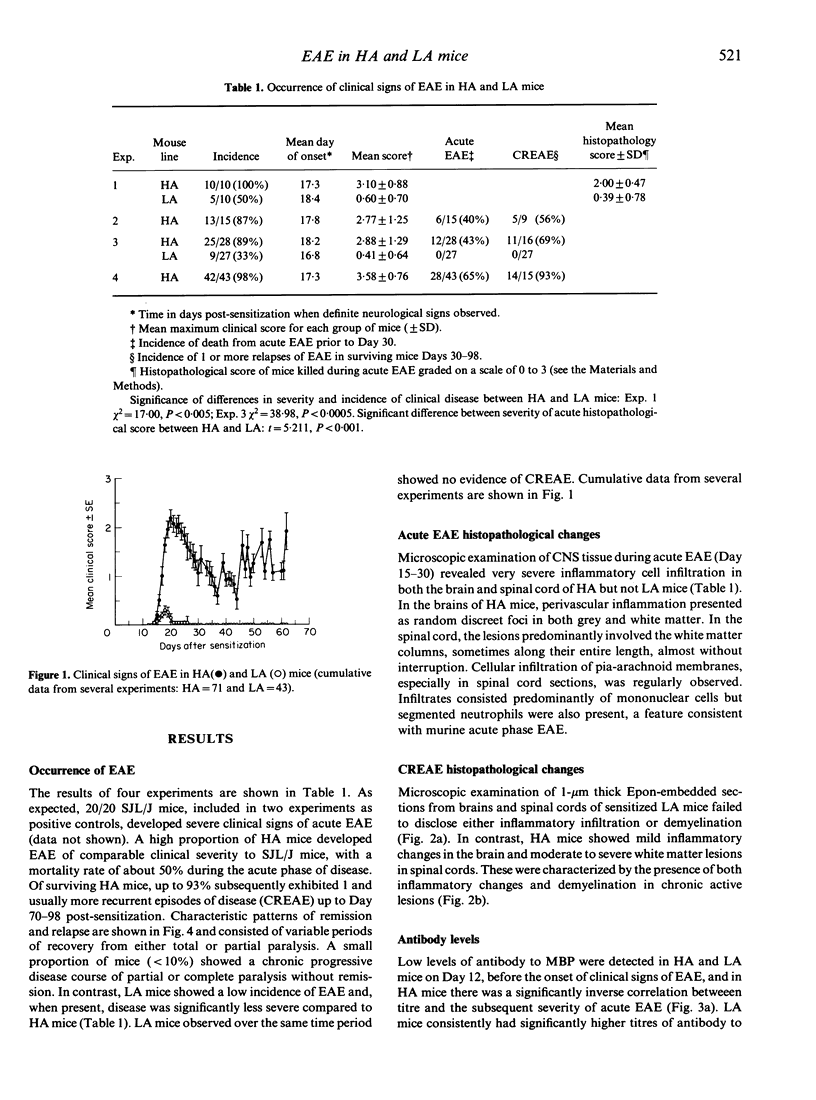
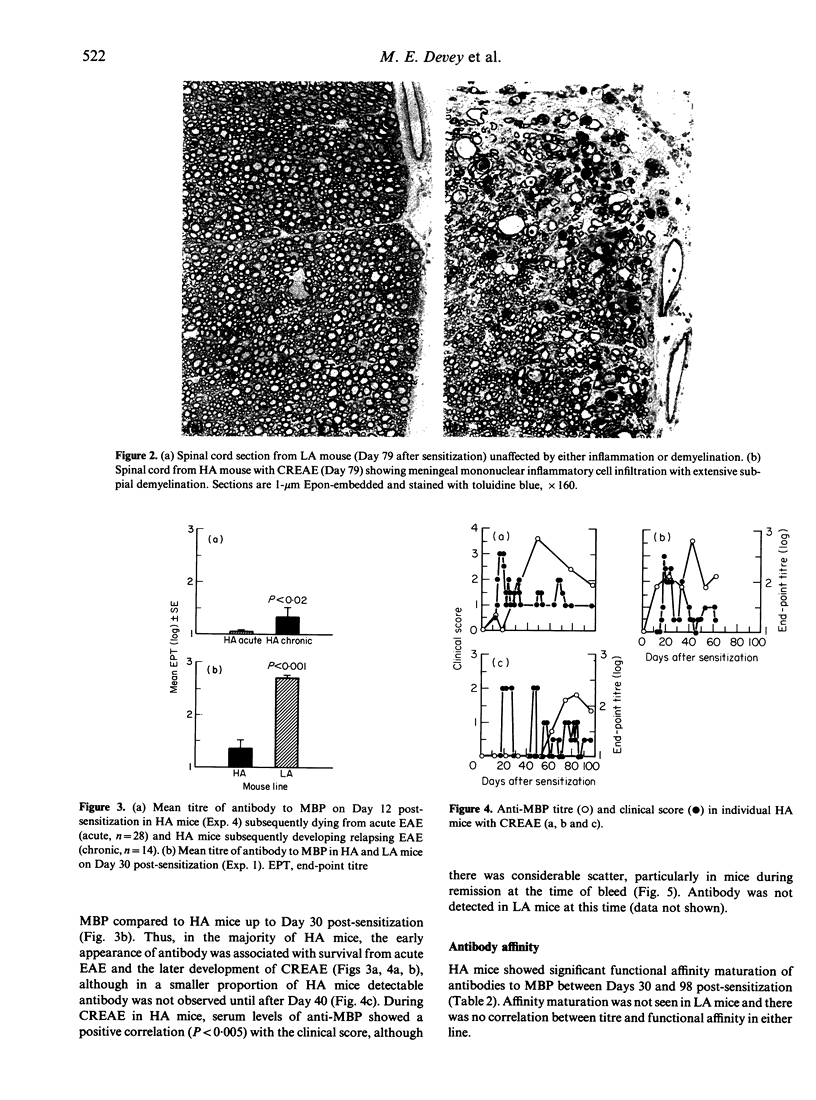
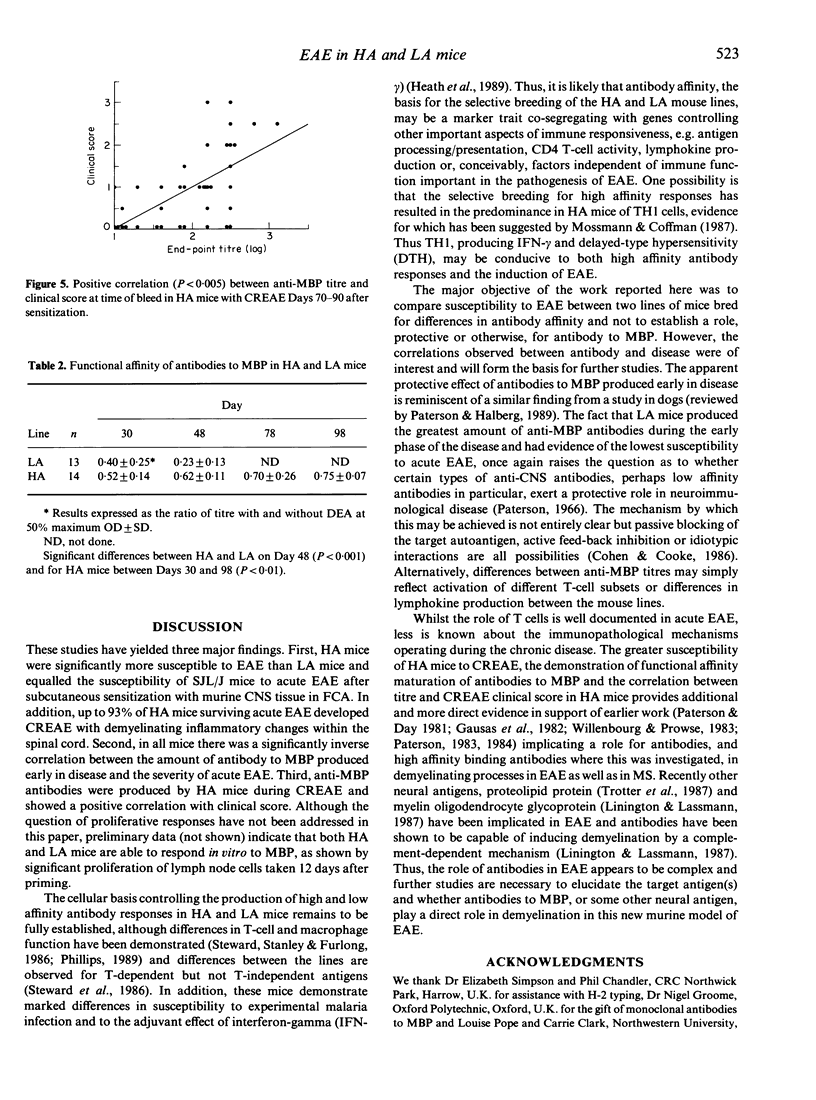
Induction of experimental allergic encephalomyelitis (EAE) in mice genetically selected to produce either high affinity (HA) or low affinity (LA) antibody responses has revealed significant differences in disease susceptibility between the two lines. HA mice were highly susceptible to EAE following subcutaneous sensitization to mouse central nervous system (CNS) tissue emulsified in Freund's complete adjuvant (FCA). Furthermore, of HA mice surviving acute EAE, up to 93% subsequently developed chronic relapsing disease (CREAE) characterized by variable demyelinating inflammatory changes within the spinal cord. In contrast, LA mice, despite having a major histocompatability complex (MHC) haplotype associated with susceptibility to EAE, were highly resistant to the disease and showed no signs of CREAE when observed for up to 100 days post-sensitization. Antibodies to myelin basic protein (MBP) were detected in both lines but rising titres of high functional affinity antibodies were only seen in HA mice. These HA and LA lines of mice provide a new approach to the study of EAE and, in particular, the role of antibody and antibody affinity in the chronic relapsing form of the disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Brostoff S. W., Mason D. W. Experimental allergic encephalomyelitis: successful treatment in vivo with a monoclonal antibody that recognizes T helper cells. J Immunol. 1984 Oct;133(4):1938–1942. [PubMed] [Google Scholar]
- Brown A. M., McFarlin D. E. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981 Sep;45(3):278–284. [PubMed] [Google Scholar]
- Carbone A. M., Ovadia H., Paterson P. Y. Role of macrophage-myelin basic protein interaction in the induction of experimental allergic encephalomyelitis in Lewis rats. J Immunol. 1983 Sep;131(3):1263–1267. [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K., Lee S., Rath S. Determination of the functional affinity of IgG1 and IgG4 antibodies to tetanus toxoid by isotype-specific solid-phase assays. J Immunol Methods. 1988 Jan 21;106(1):119–125. doi: 10.1016/0022-1759(88)90279-7. [DOI] [PubMed] [Google Scholar]
- Gausas J., Paterson P. Y., Day E. D., Dal Canto M. C. Intact B-cell activity is essential for complete expression of experimental allergic encephalomyelitis in Lewis rats. Cell Immunol. 1982 Sep 15;72(2):360–366. doi: 10.1016/0008-8749(82)90484-1. [DOI] [PubMed] [Google Scholar]
- Heath A. W., Devey M. E., Brown I. N., Richards C. E., Playfair J. H. Interferon-gamma as an adjuvant in immunocompromised mice. Immunology. 1989 Aug;67(4):520–524. [PMC free article] [PubMed] [Google Scholar]
- Katz F. E., Steward M. W. The genetic control of antibody affinity in mice. Immunology. 1975 Sep;29(3):543–548. [PMC free article] [PubMed] [Google Scholar]
- Linington C., Lassmann H. Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol. 1987 Dec;17(1):61–69. doi: 10.1016/0165-5728(87)90031-2. [DOI] [PubMed] [Google Scholar]
- PATERSON P. Y. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960 Jan 1;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson P. Y., Day E. D. Current perspectives of neuroimmunologic disease: multiple sclerosis and experimental allergic encephalomyelitis (1,2). Clin Immunol Rev. 1981;1(4):581–697. [PubMed] [Google Scholar]
- Paterson P. Y. Experimental allergic encephalomyelitis and autoimmune disease. Adv Immunol. 1966;5:131–208. doi: 10.1016/s0065-2776(08)60273-4. [DOI] [PubMed] [Google Scholar]
- Paterson P. Y. LT/EAE and the MS quest. Going to the dogs and rats to study the patient. Cell Immunol. 1983 Nov;82(1):55–74. doi: 10.1016/0008-8749(83)90140-5. [DOI] [PubMed] [Google Scholar]
- Phillips C. Prostaglandin E2 production is enhanced in mice genetically selected to produce high affinity antibody responses. Cell Immunol. 1989 Apr 1;119(2):382–392. doi: 10.1016/0008-8749(89)90252-9. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Barnett L. B., Brown A., Behar T., McFarlin D. E. Neuropathology of experimental allergic encephalomyelitis in inbred strains of mice. Lab Invest. 1980 Aug;43(2):150–157. [PubMed] [Google Scholar]
- Sindic C. J., Cambiaso C. L., Masson P. L., Laterre E. C. The binding of myelin basic protein to the Fc region of aggregated IgG and to immune complexes. Clin Exp Immunol. 1980 Jul;41(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Reinhardt M. C., Staines N. A. The genetic control of antibody affinity. Evidence from breeding studies with mice selectively bred for either high or low affinity antibody production. Immunology. 1979 Jul;37(3):697–703. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Stanley C., Furlong M. D. Antibody affinity maturation in selectively bred high and low-affinity mice. Eur J Immunol. 1986 Jan;16(1):59–63. doi: 10.1002/eji.1830160112. [DOI] [PubMed] [Google Scholar]
- Swanborg R. H., Swierkosz J. E., Saieg R. G. Studies on the species-variability of experimental allergic encephalomyelitis in guinea pigs and rats. J Immunol. 1974 Feb;112(2):594–600. [PubMed] [Google Scholar]
- Trotter J. L., Clark H. B., Collins K. G., Wegeschiede C. L., Scarpellini J. D. Myelin proteolipid protein induces demyelinating disease in mice. J Neurol Sci. 1987 Jun;79(1-2):173–188. doi: 10.1016/0022-510x(87)90271-1. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Prowse S. J. Immunoglobulin-deficient rats fail to develop experimental allergic encephalomyelitis. J Neuroimmunol. 1983 Oct;5(2):99–109. doi: 10.1016/0165-5728(83)90001-2. [DOI] [PubMed] [Google Scholar]



