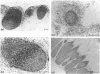
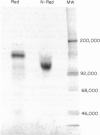
Abstract
A new bovine B-cell differentiation antigen is described that is detected by three monoclonal antibodies (mAb). The antigen is not an immunoglobulin and is precipitated from peripheral B cells as a molecule with an approximate molecular weight (MW) of 120,000 or 145,000 before and after reduction, respectively. Data obtained from two-colour cytofluorimetry and immunohistochemistry confirmed that the antigen was found only on mature B cells and on cells with dendritic morphology in the follicles of the organized lymphoid tissues. Its level of expression is directly correlated with that of IgM on peripheral blood B cells and Theileria parva-transformed B cells. The marker was also expressed on the peripheral cells which expressed surface IgG. Based on the antigen's cellular distribution, biochemistry and histochemistry, it is considered to be analogous to the human CD21 antigen.
Full text
PDF

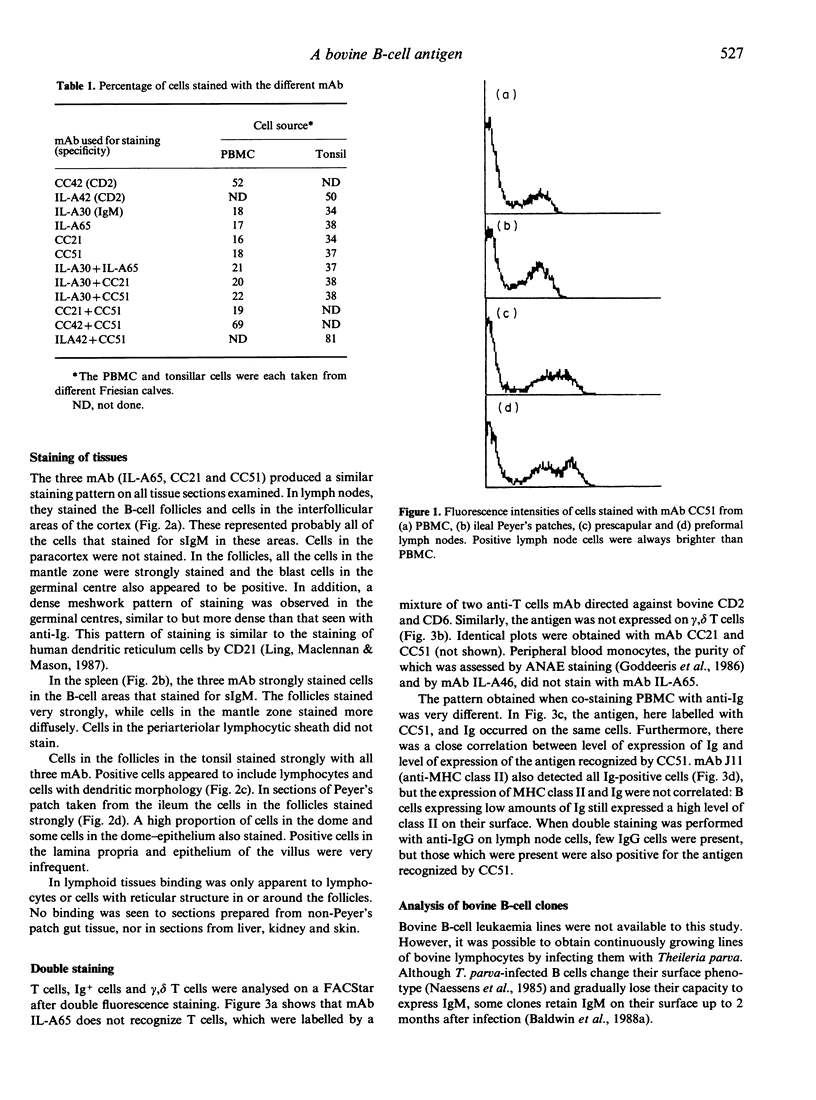
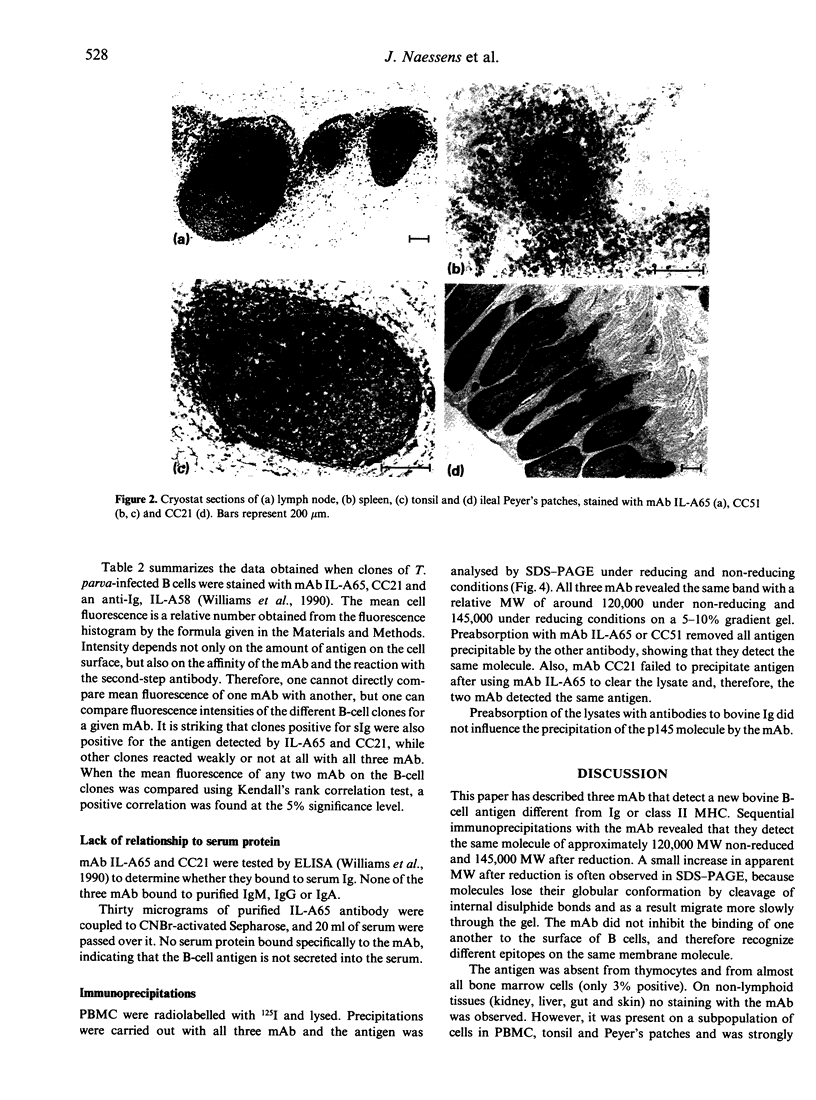

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. L., Black S. J., Brown W. C., Conrad P. A., Goddeeris B. M., Kinuthia S. W., Lalor P. A., MacHugh N. D., Morrison W. I., Morzaria S. P. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect Immun. 1988 Feb;56(2):462–467. doi: 10.1128/iai.56.2.462-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. L., Machugh N. D., Ellis J. A., Naessens J., Newson J., Morrison W. I. Monoclonal antibodies which react with bovine T-lymphocyte antigens and induce blastogenesis: tissue distribution and functional characteristics of the target antigens. Immunology. 1988 Mar;63(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- Davis W. C., Ellis J. A., MacHugh N. D., Baldwin C. L. Bovine pan T-cell monoclonal antibodies reactive with a molecule similar to CD2. Immunology. 1988 Jan;63(1):165–167. [PMC free article] [PubMed] [Google Scholar]
- Davis W. C., Marusic S., Lewin H. A., Splitter G. A., Perryman L. E., McGuire T. C., Gorham J. R. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet Immunol Immunopathol. 1987 Jul;15(4):337–376. doi: 10.1016/0165-2427(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Goddeeris B. M., Baldwin C. L., ole-MoiYoi O., Morrison W. I. Improved methods for purification and depletion of monocytes from bovine peripheral blood mononuclear cells. Functional evaluation of monocytes in responses to lectins. J Immunol Methods. 1986 May 22;89(2):165–173. doi: 10.1016/0022-1759(86)90354-6. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Parsons K. R., Jones B. V., Sopp P., Pocock D. H. Two monoclonal antibodies (CC17, CC29) recognizing an antigen (Bo5) on bovine T lymphocytes, analogous to human CD5. Vet Immunol Immunopathol. 1988 Sep;19(2):127–139. doi: 10.1016/0165-2427(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Iida K., Nadler L., Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983 Oct 1;158(4):1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunita S., Koyama H., Saito H. Preparation and characterization of monoclonal antibodies against bovine B lymphocyte surface antigens. Vet Immunol Immunopathol. 1988 Apr;18(3):201–212. doi: 10.1016/0165-2427(88)90064-5. [DOI] [PubMed] [Google Scholar]
- Lewin H. A., Davis W. C., Bernoco D. Monoclonal antibodies that distinguish bovine T and B lymphocytes. Vet Immunol Immunopathol. 1985 May;9(1):87–102. doi: 10.1016/0165-2427(85)90132-1. [DOI] [PubMed] [Google Scholar]
- MacHugh N. D., Bensaid A., Davis W. C., Howard C. J., Parsons K. R., Jones B., Kaushal A. Characterization of a bovine thymic differentiation antigen analogous to CD1 in the human. Scand J Immunol. 1988 May;27(5):541–547. doi: 10.1111/j.1365-3083.1988.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Naessens J., Newson J., Bensaid A., Teale A. J., Magondu J. G., Black S. J. De novo expression of T cell markers on Theileria parva-transformed lymphoblasts in cattle. J Immunol. 1985 Dec;135(6):4183–4188. [PubMed] [Google Scholar]
- Naessens J., Newson J., Williams D. J., Lutje V. Identification of isotypes and allotypes of bovine immunoglobulin M with monoclonal antibodies. Immunology. 1988 Apr;63(4):569–574. [PMC free article] [PubMed] [Google Scholar]
- Pinder M., Pearson T. W., Roelants G. E. The bovine lymphoid system: II. Derivation and partial characterlzation of monoclonal antibodies against bovine peripheral blood lymphocytes. Vet Immunol Immunopathol. 1980 Dec;1(4):303–316. doi: 10.1016/0165-2427(80)90010-0. [DOI] [PubMed] [Google Scholar]
- Siaw M. F., Nemerow G. R., Cooper N. R. Biochemical and antigenic analysis of the Epstein Barr virus/C3d receptor (CR2). J Immunol. 1986 Jun 1;136(11):4146–4151. [PubMed] [Google Scholar]