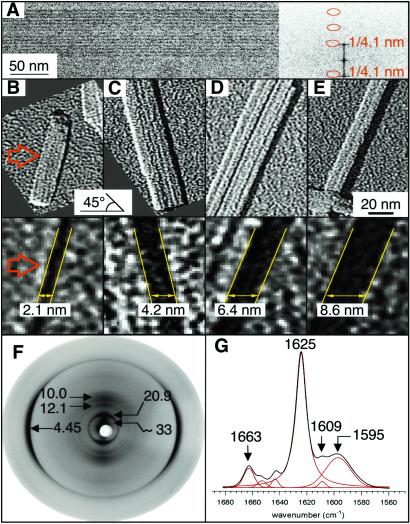
Fig 3.
Structural characterization of the fibrils formed by peptide no. 3 (STVIIE) at pH 2.6 by cryo-EM, unidirectional metal shadowing, x-ray fiber diffraction, and FTIR measurements. (A) The center-to-center spacing of the protofilament was determined by cryo-EM. (B–E) The height of flattened amyloid fibrils was determined by high resolution surface shadowing. The elevation angle of the evaporation is 45°, and the azimuthal angle is horizontal (arrow in B). Accordingly, the length of the shadow in the azimuthal direction correlates directly with the height of the fibrils. The smallest (mono-) layers have been found to be in the order of 21 Å (B). In addition, these layers or “ribbons” can stack one on top of another to give multiples of 21 Å such as double (C), triple (D), or even quadruple (E) layers. (F) X-ray diffraction pattern from oriented fibers showing the reflections that define the organization of the protofilaments. One more reflection on the equator can be observed at 5.65 ± 0.03 Å that could arise from a harmonic of either 10.0- or 12.1-Å diffractions (or a combination of both). (G) FTIR amide I band of the fibrils indicating the frequencies of its main components.