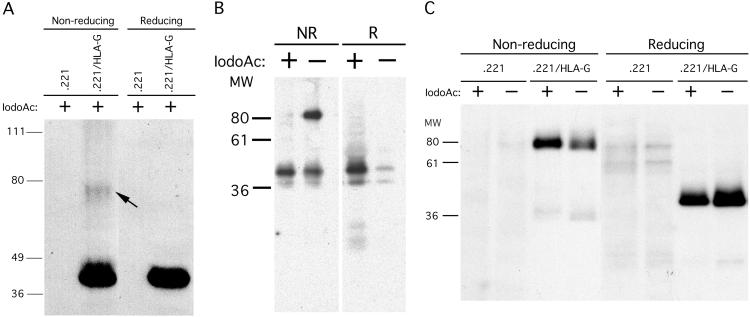
Fig 3.
Cell-surface dimerization of HLA-G. (A) 721.221/HLA-G transfectants and the 721.221 parental cell line were lysed in SDS/PAGE buffer containing iodoacetamide and run under nonreducing and reducing conditions. After blotting to nitrocellulose, HLA-G heavy chains were detected by using the HLA-G-specific mAb, MEM-G/1. (B) Immunoprecipitation of cell surface-biotinylated HLA-A2 molecules from 721.221/HLA-A2 transfectants. Inclusion of iodoacetamide abrogated the formation of HLA-A2 dimer artifacts, because under nonreducing conditions the ≈85-kDa MM band does not appear in samples lysed in the presence of iodoacetamide. (C) Cell-surface molecules of 721.221/HLA-G transfectants and the 721.221 parental cell line were biotinylated, and cells were lysed in the presence or absence of iodoacetamide. Class I MHC molecules were immunoprecipitated with BBM.1 and run under both nonreducing and reducing conditions. Even in the presence of iodoacetamide, HLA-G dimers were detected.