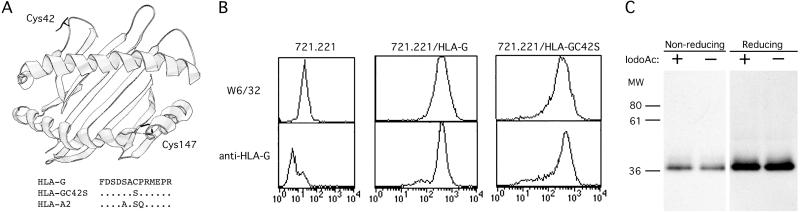
Fig 4.
Identification and mutagenesis of extracellular cysteines in HLA-G. (A) A ribbon diagram of the crystal structure of HLA-A2 with Cys-42 and Cys-147 residues superimposed. Shown below is a portion of the α1 domain HLA-G sequence containing Cys-42 which was chosen for mutagenesis to a serine. Dots (.) indicate the residue identity with the HLA-G sequence. (B) Cell-surface expression of HLA-G/C42S. 721.221 B-LCLs transfected with HLA-G/C42S were stained with the conformation-specific mAb W6/32 and the HLA-G-specific mAb, MEM-G/11. After FACS-sorting, HLA-G/C42S and the wild-type HLA-G transfectants expressed similar levels of protein. (C) Mutagenesis of Cys-42 to Ser-42 completely abrogates HLA-G dimerization. HLA-G/C42S transfectants were cell surface-biotinylated and lysed in the presence or absence of iodoacetamide. Class I MHC molecules were immunoprecipitated and run under both nonreducing and reducing conditions. Even in the absence of iodoacetamide, HLA-G dimers could not be detected.