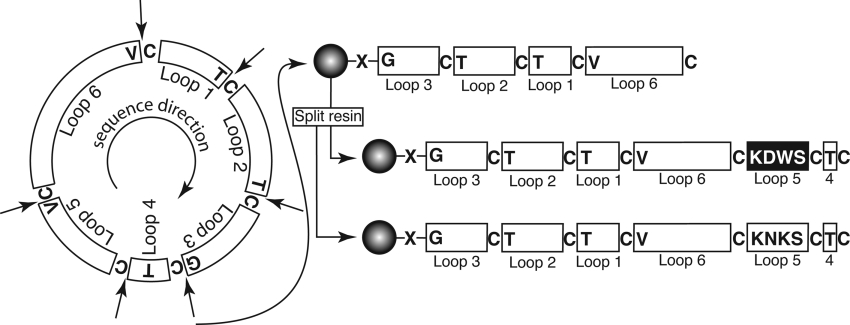
Figure 2. Schematic illustration of the synthetic strategy utilized for grafted cyclotide analogues.
There are six possible starting points for the synthesis, at the N-terminal side of each of the cysteine residues, which are indicated by arrows; the amino acid that would become the C-terminal residue in the linear precursor is also shown. The two modified kalata B1 analogues described in the present study were synthesized using solid-phase methods by starting at the glycine residue in loop 3, as shown on the right, and attached to the resin via a thioester linker (X). The peptide chain was built up on-resin (starting at the C-terminus) until loop 5 was reached, when the resin was split and the synthesis of the two analogues completed separately. After cleavage of the peptide chain from the resin, cyclization and disulphide formation was done simultaneously by incubating the peptides in aqueous buffer at pH 8.5.