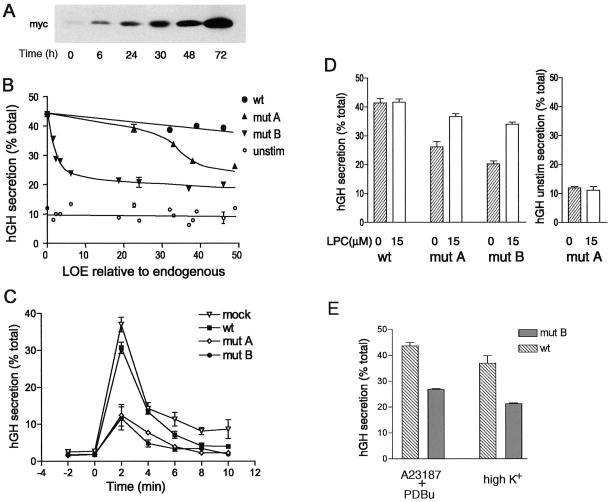
Figure 6.
Tet-regulated expression of myc-tagged SCAMP2 and its mutants in PC12 cells and dose-dependent inhibition of depolarization-induced secretion of hGH. (A) Western blots with anti-myc antibody 9E10 illustrating the change in expression of exogenous SCAMP (mutant A used as an example) when dox is added at specified times after transfection. (B) Plot of hGH secretion (percentage of total) stimulated by depolarization (●, ▴, ▾) or unstimulated (○) as a function of the level of expression (LOE) of exogenous SCAMP2 relative to endogenous SCAMP2. Secretion of hGH was determined by ELISA. Mock transfection (pTRE vector) is shown at zero LOE with 42% hGH secretion (32% net stimulated). The data are compiled from eight independent transfection experiments. Error bars indicate the range obtained when independent experiments resulted in the same LOE relative to endogenous. (C) Time course of depolarization-induced hGH secretion by PC12 cells that had been transfected with pTRE2 vector, wild-type SCAMP2, mutant A, or mutant B. Samples of tet-regulated PC12 cells cotransfected in duplicate with cDNAs encoding hGH and either empty pTRE2 vector (mock), wild-type (wt) SCAMP2, mutant (mut) A, or mutant B were treated with 2 μg/ml dox at times that achieve comparable levels of expression. At 72 h after transfection, the samples were used to assay the rate of secretion at 2-min intervals. High-K+ medium was added at 0 min to initiate depolarization. (D) Addition of LPC to the medium substantially overcomes the inhibition of secretion caused by overexpression of SCAMP2 mutants A and B. Samples of tet-regulated PC12 cells cotransfected in duplicate with cDNAs encoding hGH and either wild-type SCAMP2, mutant A, or mutant B were treated with 2 μg/ml dox at times that achieve comparable levels of expression. At 72 h after transfection, the samples were incubated 10 min in low-K+ medium and then 10 min in high-K+ medium in the absence or presence of 15 μM LPC, and the media and cell lysates were assayed for hGH by ELISA and expressed as percentage of total. The results are from five independent experiments. For hGH unstimulated secretion (right), the results for mutant A are plotted as an example. For wild-type SCAMP2, the results are identical; for mutant B, hGH unstimulated secretion is 14.5 and 12% for 0 and 15 μM LPC, respectively. Error bars indicate mean ± SEM. (E) Overexpression of mutant B inhibits secretion of hGH stimulated by a combination calcium ionophore A23187 and phorbol dibutyrate. Samples of tet-regulated PC12 cells were cotransfected with cDNAs encoding hGH and either wild-type SCAMP2 or mutant B and were induced exactly as in D. Duplicate samples in three independent experiments were incubated 10 min in low-K+ medium (with or without dimethyl sulfoxide, the solvent for A23187 and phorbol dibutyrate; data with dimethyl sulfoxide shown) and then 10 min in either low-K+ medium with 0.5 μM A23187 and 0.1 μM phorbol dibutyrate (PDBu) or high-K+ medium. Media and cell lysates were assayed for hGH, and results are expressed as in D. Unstimulated secretion of hGH was 12–15% of total in all cases.