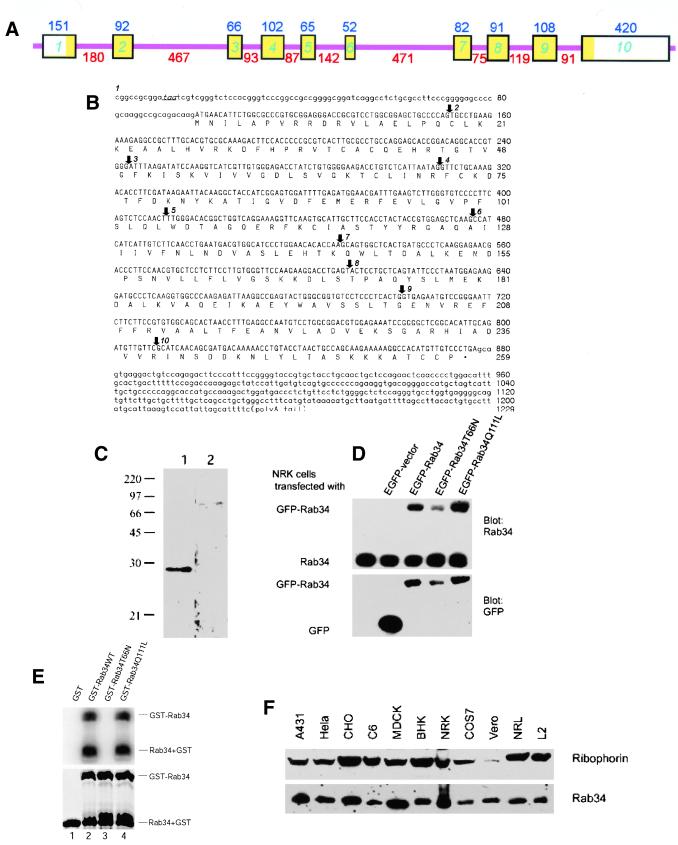
Figure 1.
Molecular and biochemical characterizations of Rab34. (A) Mouse Rab34 gene organization: the boxes indicate various exons interrupted by the introns depicted as thin pink lines. The sizes of the exons are indicated above each box, and those of introns are indicated below each intron. The exon regions corresponding to the open reading frame are indicated in yellow. (B) Nucleotide and deduced amino acid sequences of mouse Rab34: The in-frame stop codon preceding the initiation cordon is indicated in italics and underlined. Arrows indicate the starting points of exon 2–10 as indicated. (C) Antibodies raised against GST-Rab34 specially recognize a 29-kDa protein. Total lysates of NRK cells were resolved by SDS-PAGE and transferred to filters. The filters were incubated with anti-Rab34 antibodies in the presence of GST (lane 1) or GST-Rab34 (lane 2). As shown, detection of the 29-kDa polypeptide was abolished by preincubation with the antigen but not GST. (D) Characterization of EGFP-Rab34 fusion proteins. Control NRK cells and cells transfected with EGFP vector, EGFP-Rab34, GDP-restricted EGFP-Rab34T66N, and GTP-restricted EGFP-Rab34Q111L as indicated were analyzed by immunoblot with anti-Rab34 antibodies (top) or anti-GFP antibodies (bottom). In addition to the endogenous 29-kDa Rab34 polypeptide, anti-Rab34 also detected the fusion proteins of ∼59 kDa. (E) Wild-type and Rab34Q111L but not Rab34T66N bind GTP as revealed by GTP overlay assay. The recombinant GST proteins resolved by SDS-PAGE were either stained with Coomassie blue (bottom) or transferred to a filter and incubated with 32P-GTP (top). In addition to the GST-Rab34, autocleavage of fusion proteins into GST and Rab34 was observed. (F) Rab34 is widely expressed in mammalian cells. Approximately 30 μg of total lysates derived from the indicated cells were analyzed by immunoblot using anti-Rab34 antibodies or antibodies against ribophorin.