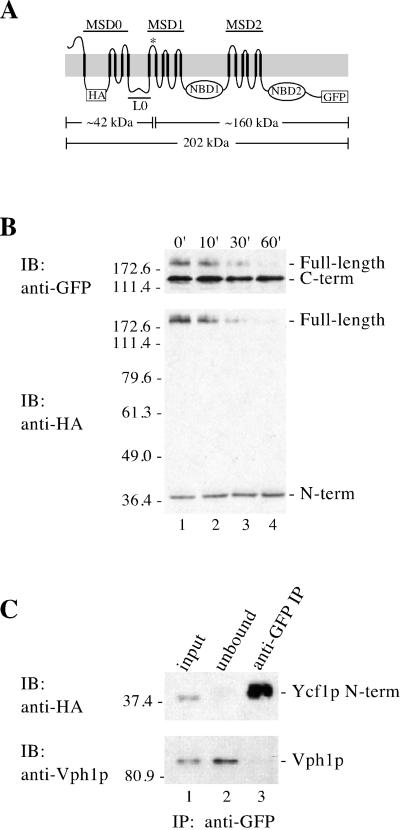
Figure 4.
Proteolytic processing of Ycf1p yields two stable products that associate with each other. (A) Schematic of Ycf1p shows the location of the potential cleavage site (*), the triple HA epitope, the GFP tag, and the approximate molecular weight of the N- and C-terminal epitope-tagged cleavage products based on SDS-PAGE migration. (B) Stability of the cleavage fragments was examined by cycloheximide chase analysis. After addition of 10 μg of cycloheximide to 10 OD600 units of mid-log cells to stop protein synthesis, aliquots were removed at the indicated times. Crude yeast cell extracts (0.5 OD600 cell equivalents per lane) were resolved by 10% SDS-PAGE and transferred to nitrocellulose. The same immunoblot was probed sequentially with two different antibodies to detect the N- and C-termini of Ycf1p-HA-GFP by using rat anti-HA monoclonal antibodies (bottom) and rabbit anti-GFP polyclonal antibodies (top), respectively. (C) Association between the N- and C-terminal cleavage products of Ycf1p was examined by coimmunoprecipitation. Crude membranes were prepared after a 60-min cycloheximide chase as described in MATERIALS AND METHODS and subjected to IP with rabbit anti-GFP polyclonal antibodies under nondenaturing conditions to pull down the C-terminal cleavage product and any associated proteins. Immunoprecipitated proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose. The presence of the N-terminal Ycf1p cleavage product in the anti-GFP IP was determined by probing the immunoblot with rat anti-HA monoclonal antibodies (top, lane 3). The N-terminal Ycf1p band detected by immunoblotting is specific, because it was not detected in a control which contains Ycf1p-GFP, but lacks the HA tag (our unpublished data). The presence or absence of an unrelated vacuolar protein, Vph1p, was assessed using mouse anti-Vph1p monoclonal antibodies (bottom, lane 3). Lanes 1, 2, and 3 contain ∼1, ∼1, and 50% of the total, respectively. The strain used for B and C is SM4542.