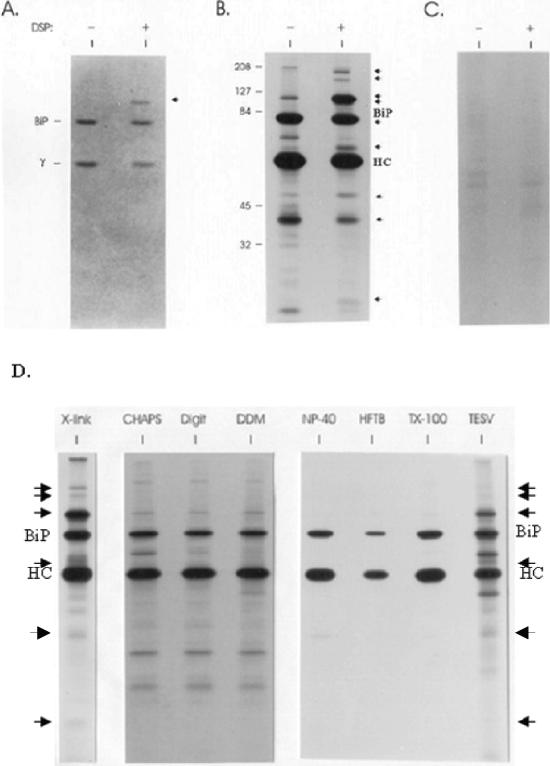
Figure 1.
Unassembled Ig heavy chains associate with a number of ER proteins in addition to BiP. The postnuclear fraction was obtained from metabolically labeled Ag8(8) cells and divided into two tubes: one for a negative control and the other treated with 150 μg/ml DSP. After quenching, vesicles were solubilized and heavy chains were precipitated with protein A-Sepharose for SDS-PAGE analysis under reducing conditions. The gel was first stained with Coomassie Blue (A) and then exposed to film for an autoradiograph (B). BiP and a γ heavy chain are indicated on the left of the gel. Additional proteins detected after cross-linking are marked with arrows. Ag8.653 cells were labeled and treated similarly (− and +DSP) and then immunoprecipitated with protein A-Sepharose (C). The cell lysates were obtained by using different detergents to release the ER proteins (1% CHAPS, 1% digitonin, 1% dodecylmaltoside [DDM], 0.5% deoxycholic acid, 0.5% NP-40, and 0.2% Triton X-100) or no detergent (a cycle of freeze-thawing followed by homogenization of the postnuclear fraction in HFTB buffer (HFTB) or 10-s sonication in TESV buffer (TESV) as described in MATERIALS AND METHODS (D). Additional proteins detected after cross-linking and after sonication are indicated with arrows.