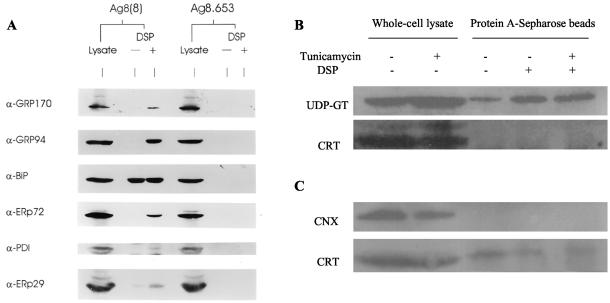
Figure 2.
Heavy chain–BiP complexes contain substantial quantities of GRP94 and smaller amounts of other chaperones and folding enzymes. Total cell lysates from 1 × 106 Ag8(8) and Ag8.653 cells were used as a positive control for the antibodies (lanes 1 and 4). The postnuclear fraction from 10 × 106 cells was either treated with DSP (lanes 3 and 6) or left untreated (lanes 2 and 5) and lysates were prepared as described in MATERIALS AND METHODS. Lysates from Ag8(8) (lanes 2 and 3) and from Ag8.653 (lanes 5 and 6) were incubated with protein A-Sepharose and precipitated proteins were electrophoresed and transferred for blotting. Segments of the nitrocellulose were reacted with the antibodies indicated and developed as described (A). Then 10 × 106 Ag8(8) cells were treated overnight with 2 (+) or 0 (−) μg/ml tunicamycin, incubated with DSP, and heavy chains were isolated from lysates with protein A-Sepharose and processed as in A. Total cell lysate from 10 × 106 cells was used as a positive control for the antibodies (B). Cells were treated as in B except that 40 × 106 Ag8(8) cells per lane were used, and only 1/10 of the lysate was used as a positive control for the antibodies (C).