Abstract
During embryogenesis, the formation of primary vascular networks occurs via the processes of vasculogenesis and angiogenesis. In uveal melanoma, vasculogenic mimicry describes the 'embryonic-like' ability of aggressive, but not nonaggressive, tumor cells to form networks surrounding spheroids of tumor cells in three-dimensional culture; these recapitulate the patterned networks seen in patients' aggressive tumors and correlates with poor prognosis. The molecular profile of these aggressive tumor cells suggests that they have a deregulated genotype, capable of expressing vascular phenotypes. Similarly, the embryonic-like phenotype expressed by the aggressive human breast cancer cells is associated with their ability to express a variety of vascular markers. These studies may offer new insights for consideration in breast cancer diagnosis and therapeutic intervention strategies.
Keywords: breast cancer, interconverted phenotype, thrombin receptor, TIE2
Introduction
Vasculogenic mimicry in aggressive human melanoma tumor cells will be reviewed, and new evidence regarding the possibility of its existence in human breast cancer cells is presented and discussed.
Molecular vasculogenic mimicry by aggressive tumor cells
It is widely assumed that tumors require a blood supply to survive, grow, and metastasize [1]. However, this concept has been inextricably linked to angiogenesis, involving the process of signaling for new blood vessel growth into a growing tumor mass [2,3,4,5]. Recently, our laboratory and collaborators have challenged this dogma [6,7] with data generated from uveal and cutaneous melanoma models. These data demonstrated that the aggressive human melanoma tumors, but not nonaggressive tumors, consist of matrix-rich networks surrounding spheroids of tumor cells, in the absence of tumor necrosis and classic angiogenesis, thus questioning the requirement of these cells for a blood supply. These studies utilized a multidisciplinary approach of investigating the pathologic indices in patient tumors that are correlated with the data derived from tumor cell lines in three-dimensional in vitro cultures, invasion assays, and microarray analysis of differential gene expression in aggressive versus nonaggressive tumor cells. Most noteworthy was the revelation that the presence of matrix-rich networks surrounding spheroids of tumor cells in primary and metastatic melanoma tumors from patients correlated with the aggressiveness of these tumors in vivo and with poor clinical outcome [8].
The cell lines derived from the aggressive and nonaggressive patient tumors were further analyzed for differences in their biologic functions in three-dimensional cultures. These assays demonstrated the remarkable recapitulation seen in situ of hollow and closed matrix-rich networks surrounding spheroids of tumor cells, but only in the aggressive tumor cell cultures. In addition, the melanoma cells that are capable of generating these networks expressed inappropriate vascular and other molecular markers that represent a combination of phenotypes. These included endothelium (tyrosine kinase with immunoglobulin and epidermal growth factor homology domains [TIE]1, an endothelial receptor kinase, plus 12 other endothelial associated genes [6]), epithelium (keratin-8 intermediate filaments), and a mesenchymal phenotype (vimentin intermediate filaments), collectively suggesting a genetic reversion to a pluripotent embryonic-like phenotype.
The unique ability of aggressive human melanoma tumor cells to generate patterned networks, similar to the patterned networks seen during embryonic vasculogenesis, and concomitantly to express vascular markers associated with endothelial cells, their precursors and other vascular cells has been termed 'vasculogenic mimicry'. However, the physiologic significance of these networks and molecular vasculogenic mimicry is unknown, and must be rigorously tested in experimental animal models.
What do we know about the ability of nonendothelial cells to function in a vascular-related capacity? There is strong evidence suggesting that human cytotrophoblasts adopt an endothelial cell phenotype as they actively participate in the dynamics of establishing the placenta and primordial microcirculation, which has been designated 'trophoblast pseudo-vasculogenesis' [9,10,11]. With respect to tumor vascularization, the possibility of the formation or lining of a microcirculation by tumor cells has been suggested by several studies, on the basis of morphologic analyses and numerous pathology reports [12,13,14,15,16,17,18,19,20,21,22,23,24,25]. In a recent review by Tímár and Tóth [26], the diagnostic and clinical significance with regard to human melanoma and breast cancer tumor cell-lined sinuses and vascular channels was presented. However, there were no experimental data to support their functional significance. Additional studies by Hashizume et al [27] suggested that, in certain mouse mammary carcinoma and RIP-Tag2 models, openings between defective endothelial cells account for tumor vessel leakiness, leading to the lining of extravascular blood lakes by tumor cells. However, some pathologists have argued that tumor cells lining channels and sinuses that contain red blood cells in a rouleau formation represents a different phenomenon from vessel leakage [26,28], which requires further examination in appropriate animal models for melanoma.
Over the past few years, there have been confounding reports from distinguished scientists that dispute the relevance of vascular density and clinical outcome in melanomas [29,30], non-small-cell lung carcinoma [31], oral cancers [32], esophageal cancers [33], aggressive prostate cancers [34], and breast cancer [35]. Further his tomorphologic evidence that some tumors may be vascularized without neo-angiogenesis, and possibly by pre-existent organ vasculature, has been reported in non-small-cell lung carcinomas [36]. Of special interest is the recent report [37**] supporting a nonangiogenic as well as an angiogenic pathway in breast cancer metastasis, which may further complicate treatment strategies. In addition, there are emerging data pointing to the expression of vascular markers, such as the thrombin receptor in breast cancer cells and tissue [38*], TIEs and angiopoietins in tumor cells of Kaposi's sarcoma [39], and vascular endothelial growth factor (VEGF) in melanoma cells and tissues [40].
Collectively, these data prompted us to conduct a preliminary molecular analysis of these vascular markers specifically expressed by aggressive versus nonaggressive breast cancer cells, in order to determine whether a pattern associated with the aggressive tumor cell phenotype might emerge. These data could provide additional markers for further diagnostic testing and therapeutic intervention strategies that might augment current anti-angiogenesis therapies.
Expression of vascular markers by breast cancer cells
Observational pathology reports and experimental data suggest that keratin (a epithelial marker) and vimentin (a mesenchymal marker) intermediate filament coexpression in both melanoma and breast cancer confers a more aggressive `interconverted' phenotype, which is suggestive of a genetic reversion to an embryonic-like cell type [41,42]. This association between the interconverted phenotype expressed by both the aggressive melanoma and breast cancer cells prompted a preliminary molecular analysis of vascular-associated markers (expressed by aggressive melanoma cells) that might be similarly expressed by aggressive breast cancer cells.
Using the nonaggressive MCF-7 and aggressive MDA-MB-231 breast cancer cell lines, molecular analysis of specific vascular markers was performed using ribonuclease protection assay, differential display, and Northern blot analyses (Fig. 1). The human umbilical vein endothelial cells (HUVECs) were used as a positive control for all of the vascular markers tested by ribonuclease protection assay. Of the vascular molecules identified, the MCF-7 cells expressed only modest amounts of VEGF, whereas MDA-MB-231 cells expressed higher levels. The highly aggressive MDA-MB-231 cells expressed thrombin receptor, TIE2, CD31, and VEGF; the expression patterns of these markers most closely resembled those in the HUVEC cells, with the exception of endoglin. Previously performed differential display analysis [43] revealed strong expression by MDA-MB-231 of an unknown gene, bc-48, which has homology in the 3' region with endothelin-B receptor, another endothelial associated marker. This expression was confirmed by Northern blot analysis, showing an intense band in MDA-MB-231 cells and no expression in MCF-7 cells. These data are further summarized in Table 1.
Figure 1.
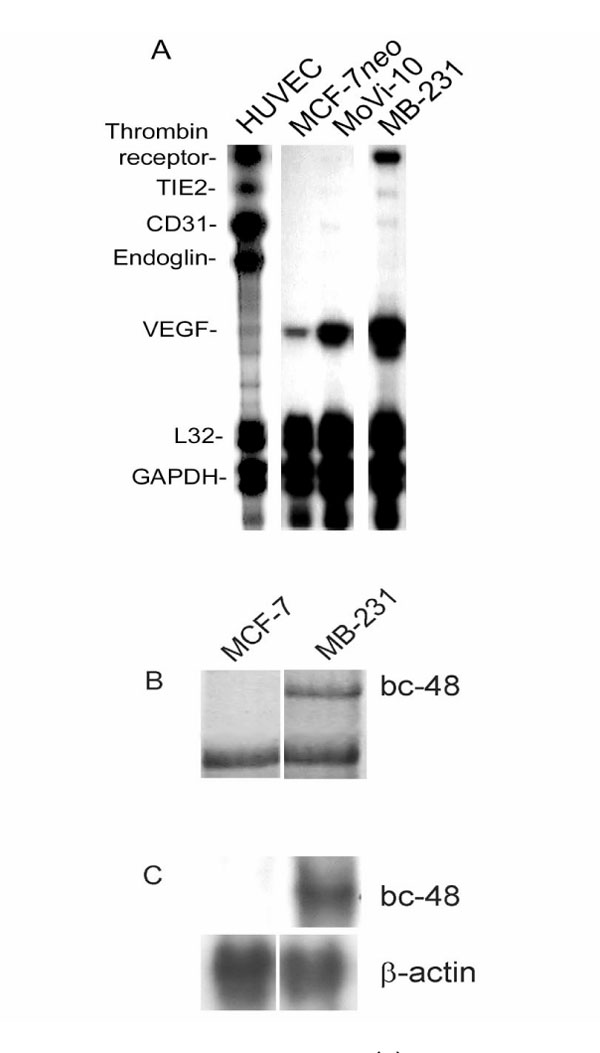
(A) Ribonuclease protection assay analysis, (B) differential display analysis, and (C) Northern blot analysis of vascular markers expressed by human breast cancer cell lines, including the following: thrombin receptor, endothelin B receptor (methods described by Kirschmann et al [43]), TIE2, CD31, endoglin and VEGF. Equal loading was assessed by ribosomal protein L32 (L32) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression (A), genes not differentially expressed (B), and β-actin (C). These data are summarized in Table 1.
Table 1.
Summary of vascular marker expression
| Human breast | Thrombin | Endothelin B | |||||||
| cancer cells | receptor | receptor | TIE-2 | CD31 | Endoglin | Angiopoietin-1 | VEGF | L32 | GAPDH |
| HUVEC | ++ | ++* | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| MCF-7 | - | - | - | - | - | - | + | ++ | ++ |
| MB-231 | ++ | ++ | + | + | - | - | +++ | ++ | ++ |
Comparison of qualitative expression assessment of vascular markers by human breast cancer cells MCF-7 (poorly aggressive), MDA-MB-231 (MB-231; highly aggressive) and HUVECs, as shown in the raw data from the ribonuclease protection assay and differential display analysis in Figure 1. Equal loading of experimental samples was achieved by ribosomal protein L32 (L32) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control lanes. *Data not shown.
The extent of vascular marker expression by the highly aggressive MDA-MB-231 cells, including thrombin receptor, TIE2, CD31, VEGF, and bc-48, has been shown to be associated with endothelial cells, as well as a few other cell types [44,45,46,47,48,49]. Of special significance is the expression by MDA-MB-231 cells of thrombin receptor, a member of the protease-activated receptor family. A previous report [44] showed that thrombin increases the invasive activity of MDA-MB-231 cells by a thrombin receptor-dependent mechanism, which may help to elucidate some of the molecular mechanisms that underlie the invasive and metastatic ability of these highly aggressive tumor cells. When thrombin receptor expression is experimentally downregulated in highly metastatic human breast cancer cells, their invasive ability is significantly diminished [38*]. Interestingly, that study also highlighted the importance of thrombin receptor expression in invading cytotrophoblasts during placental implantation of the human embryo [38*]. Indeed, others have shown that one of the mechanisms of thrombin-induced angiogenesis is the potentiation of VEGF activity on endothelial cells via the upregulation of VEGF receptors [47], which may help to explain the simultaneous increase in both VEGF and thrombin receptor by MDA-MB-231 cells demonstrated in this molecular survey.
VEGF is a critical factor in vasculogenesis and angiogenesis, and has been shown [40] to be expressed by melanoma cells in a majority of metastases. TIE2, VEGF, and CD31, collectively, have also been demonstrated in other carcinoma cells, such as human nonsmall-cell lung carcinomas, and are considered important angiogenic factors [48]. The upregulation of bc-48 (by MDA-MB-231 cells), a putative endothelin-B receptor, has heretofore been associated with vascular tube formation by endothelial cells [49], and lends further support to the concept that highly aggressive breast cancer cells are capable of expressing vascular markers previously thought to be associated only with endothelial cells, their precursors, and other vascular cells. Taken together, this preliminary molecular screen suggests for the first time that aggressive breast cancer cells express a diverse subset of vascular markers, whose clinical and pathophysiological significance requires further examination.
Significance of putative vasculogenic mimicry in cancer
During embryogenesis, the formation of primary vascular networks occurs via the processes of vasculogenesis and angiogenesis. During tumorigenesis, it is tempting to speculate that both of these processes are recapitulated to provide the requisite nutritional supply to growing tumors. Certainly, this has been proven and accepted for angiogenesis, and there is a growing body of circumstantial in vitro and molecular evidence to suggest that vasculogenesis may play a putative role as well. There is additional in vivo evidence in patients' tumors that supports nonangiogenic pathways with possible cooption of the existing vasculature in both nonsmall-cell lung carcinoma and breast cancer metastasis [36,37**].
In uveal melanoma, the evolving concept of vasculogenic mimicry describes the 'embryonic-like' ability of aggressive, but not nonaggressive tumor cells to form channels within matrix-rich networks surrounding spheroids of tumor cells in three-dimensional culture, which recapitulates the patterned networks seen in patients' aggressive tumors and correlates with poor prognosis. The molecular profile of these aggressive tumor cells suggests that they have a deregulated genotype that is capable of expressing multiple molecular phenotypes simultaneously, particularly vascular markers associated primarily with endothelial cells and their precursors. The deregulated genotype also suggests that the aggressive melanoma tumor cells have essentially reverted to a pluripotent embryonic-like cell that is capable of transdifferentiation.
Similarly, the interconverted, embryonic-like phenotype expressed by the aggressive, but not by the nonaggressive, human breast cancer cells is associated with their ability to express a variety of vascular markers, at the molecular level. However, further evidence regarding the biologic significance of these markers in both breast cancer and melanoma is required before specific conclusions can be drawn. Interestingly, Drosophila researchers have recently referred to tumor vasculogenic mimicry channels in aggressive, fast-growing tumors containing mutations in the lats tumor suppressor gene [50], which they speculate may be important for perfusion. The Drosophila model does not have blood flow or endothelial cells; however, these tumors may hold quintessential clues regarding a potential hardwired genetic program that has been evolutionarily conserved, which needs to be further investigated to elucidate alternative, nonangiogenic pathways for tumor perfusion.
Obviously, there are more questions than answers at this time regarding the putative functional significance of vascular marker expression by aggressive melanoma and breast cancer cells. It will be important to address whether aggressive breast cancer cells form similar patterned networks in culture and in vivo, as are seen in melanoma. If tumor vasculogenesis can be demonstrated in experimental models, does it occur concomitantly with angiogenesis or as a remodeling of angiogenesis in aggressive tumors? Is pre-existing vessel cooption involved? Is tumor cell vasculogenesis an alternative angiogenic switch in aggressive tumors? Regardless of the terminology that is used to describe the expression and mimicry of vascular-like genes by aggressive tumor cells, this area of research merits further exploration, with potential benefits for molecular diagnosis and therapeutic intervention strategies. Carmeliet [51], in a review of the mechanisms that underlie angiogenesis and arteriogenesis, speculated that the recent controversial findings of mosaic tumor vessels and vasculogenic tumor cells (vasculogenic mimicry) "may have considerable consequences for antiangiogenesis tumor therapy".
Conclusion
There is a growing body of evidence suggesting that vasculogenic mimicry occurs in melanoma, and possibly other cancers as well; however, the significance of this finding remains under investigation. New evidence regarding the possible existence of vasculogenic mimicry in aggressive human breast cancer cells has been presented. We hope that our paper will encourage other investigators to examine breast tumors and other cancers for the putative biological significance of the molecular expression of vascular markers in aggressive cancers cells.
Acknowledgments
Acknowledgement
The authors gratefully acknowledge the invaluable scientific contributions of Drs Zhila Ellis (University of Iowa) and Katrina Trevor (University of Arizona). Research supported by NIH/NCI CA59702 (MJCH) and CA83137 (REBS).
References
- Folkman J. Clinical applications of research on angiogenesis. Seminars in Medicine of the Beth Israel Hospital, Boston. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Weidner N. Tumoral vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol. 1998;184:119–122. doi: 10.1002/(SICI)1096-9896(199802)184:2<119::AID-PATH17>3.3.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rak J, Kerbel RS. Treating cancer by inhibiting angiogenesis: new hopes and potential pitfalls. Cancer Metastas Rev. 1996;15:231–236. doi: 10.1007/BF00437476. [DOI] [PubMed] [Google Scholar]
- Kumar R, Fidler IJ. Angiogenic molecules and cancer metastasis. . In Vivo. 1998;12:27–34. [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe'er J, Trent JM, Meltzer PS, Hendrix MJC. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ. Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch: a rose by any other name? Commentary. . Science. 1999;155:675–679. doi: 10.1016/S0002-9440(10)65164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe'er J, Gruman LM. The prognostic value of tumor blood vessels morphology in primary uveal melanoma. Ophthalmology. 1993;100:1389–1398. doi: 10.1016/s0161-6420(93)31470-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype: one cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10:660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- Warren BA, Shubik P. The growth of the blood supply to melanoma transplants in the hamster cheek pouch. Lab Invest. 1966;15:464–478. [PubMed] [Google Scholar]
- Hammersen F, Endrich B, Messmer K. The fine structure of tumor blood vessels. I. Participation of non-endothelial cells in tumor angiogenesis. Int J Microcirc Clin Exp. 1985;4:31–43. [PubMed] [Google Scholar]
- Nasu R, Kimura H, Akagi K, Murata T, Tanaka Y. Blood flow influences vascular growth during tumor angiogenesis. Br J Cancer . 1999;79:780–786. doi: 10.1038/sj.bjc.6690125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis RA. Pathology of Tumours London, Butterworth and Co, Ltd . 1948.
- François J. Malignant melanomata of the choroid. Montgomery Memorial Lecture 1961. Br J Ophthalmol. 1963;47:736–743. doi: 10.1136/bjo.47.12.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J, Neetens A. Physico-anatomical studies of spontaneous and experimental intraocular newgrowths: vascular supply. Bibl Anat . 1967;9:403–411. [PubMed] [Google Scholar]
- Jensen AO. Malignant melanoma of the choroid of a peculiar cavernous type. Arch Ophthalmol. 1964;72:337–340. doi: 10.1001/archopht.1964.00970020337009. [DOI] [PubMed] [Google Scholar]
- Jensen OA. The 'Knapp-Rønne' type of malignant melanoma of the choroid. A haemangioma-like melanoma with a typical clinical picture. So-called 'preretinal malignant choroidal melanoma'. Acta Ophthalmol (Copenh) 1976;54:41–54. doi: 10.1111/j.1755-3768.1976.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Warren BA. The vascular morphology of tumors. Tumor Blood Circulation. Edited by Peterson H-I. Boca Raton: CRC Press, Inc, 1979. pp. 1–48.
- Prause JU, Jensen OA. Scanning electron microscopy of frozen-cracked, dry-cracked and enzyme-digested tissue of human malignant choroidal melanomas. Albrecht Von Graefes Arch Klin Exp Ophthalmol . 1980;212:217–225. doi: 10.1007/BF00410517. [DOI] [PubMed] [Google Scholar]
- Konerding MA, Steinberg F, Streffer C. The vasculature of xeno-transplanted human melanomas and sarcomas on nude mice. II. Scanning and transmission electron microscopic studies. Acta Anat (Basel) 1989;136:27–33. doi: 10.1159/000146793. [DOI] [PubMed] [Google Scholar]
- Jain RK. Determinants of tumor blood flow: a review. . Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. . Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- Goodall CM, Feldman R, Sanders AG, Shubik P. Vascular patterns for four transplantable tumors in the hamster (Mesocricetus auratus). Angiology. 1965;16:622–625. doi: 10.1177/000331976501601008. [DOI] [PubMed] [Google Scholar]
- Tímár J, Tóth J. Tumor sinuses: vascular channels. . Pathol Oncol Res. 2000;6:83–86. doi: 10.1007/BF03032354. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folberg R, Hendrix MJC, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Rivers J, Kerbel RS, Stankiewicz KS, White WL. Extent of vascularization as a prognostic indicator in thin (<0.76 mm) malignant melanomas. Am J Pathol. 1994;145:510–514. [PMC free article] [PubMed] [Google Scholar]
- Lane AM, Egan KM, Gradoudas ES, Yang J, Saornil MA, Alroy J, Albert D. An evaluation of tumour vascularity as a prognostic indicator in uveal melanoma. Melanoma Res. 1997;7:237–242. doi: 10.1097/00008390-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Chandrachud LM, Pendleton N, Chisholm DM, Horan MA, Schor AM. Relationship between vascularity, age and survival in non small-cell lung cancer. Br J Cancer. 1997;76:1367–1375. doi: 10.1038/bjc.1997.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich LL, Biddinger PW, Duperier FD, Gluckman JL. Tumor angiogenesis as a prognostic indicator in T2-T4 oral cavity squamous cell carcinoma: a clinical-pathologic correlation. Head Neck. 1997;19:276–280. doi: 10.1002/(SICI)1097-0347(199707)19:4<276::AID-HED5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sarbia M, Bittinger F, Porschen R, Dutkowski P, Willers R, Gabbert HE. Tumor vascularization and prognosis in squamous cell carcinomas of the esophagus. Anticancer Res. 1996;16:2117–2121. [PubMed] [Google Scholar]
- Rubin MA, Buyyounouski M, Bagiella E, Sharir S, Neugut A, Benson M, de la Taille A, Katz AE, Olsson CA, Ennis RD. Microvessel density in prostate cancer: lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology. 1999;53:542–547. doi: 10.1016/S0090-4295(98)00561-5. [DOI] [PubMed] [Google Scholar]
- Pendleton N, Pazouki S, Keerkens E, Smither RL, Chisholm DM, Moore JV, Howell A, Horan MA, Schor AM. Relationships between different measurements of vascularity and clinico-pathological parameters in breast cancer. Anticancer Res. 1998;18:4565–4568. [PubMed] [Google Scholar]
- Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S, Buyse M, Pilotti S, Pierotti M, Rilke R. Non-small-lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151:1417–1423. [PMC free article] [PubMed] [Google Scholar]
- Pezzella F, Manzotti M, Di Bacco A, Viale G, Nicholson AG, Price R, Ratcliffe C, Pastorino U, Gatter KC, Harris AL, Altman DG, Pilotti S, Veronesi U. Evidence for novel non-angiogenic pathway in breast-cancer metastasis. Lancet. 2000;355:1787–1788. [PubMed] [Google Scholar]
- Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R. Thrombin receptor overexpression in malignant and physiological invasion processes. . Nature Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD. Expression of tie1, tie2, and angiopoietins 1, 2, and 4 in Kaposi's sarcoma and cutaneous angiosarcoma. Am J Pathol. 2000;156:2179–2183. doi: 10.1016/S0002-9440(10)65088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birck A, Kirkin AF, Zeuthen J, Hou-Jensen K. Expression of basic fibroblast growth factor and vascular endothelial growth factor in primary and metastatic melanoma from the same patient. Melanoma Res. 1999;9:375–381. doi: 10.1097/00008390-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Seftor REB, Trevor KT. Experimental co-expression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am J Pathol. 1997;150:483–495. [PMC free article] [PubMed] [Google Scholar]
- Thomas PA, Kirschmann DA, Cerhan JR, Folberg R, Seftor EA, Sellers TA, Hendrix MJC. Association between keratin and vimentin expression, malignant phenotype, and survival in postmenopausal breast cancer patients. . Clin Cancer Res. 1999;5:2698–2703. [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Nieva DRC, Mariano EA, Hendrix MJC. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Br Cancer Res Treat. 1999;55:127–136. doi: 10.1023/a:1006188129423. [DOI] [PubMed] [Google Scholar]
- Henrikson KP, Salazar SL, Fenton JW, II, Pentecost BT. Role of thrombin receptor in breast cancer invasiveness. Br J Cancer. 1999;79:401–406. doi: 10.1038/sj.bjc.6690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer EM, Berger G, Berndt MC. Platelet alpha-granule and plasma membrane share two new components: CD9 and PECAM-1. Blood . 1994;84:1722–1730. [PubMed] [Google Scholar]
- Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–30905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- Tsopanoglou NE, Maragoudakis ME. On the mechanism of thrombin-induced angiogenesis. Potentiation of vascular endothelial growth factor activity on endothelial cells by up-regulation of its receptors. . J Biol Chem. 1999;274:23969–23976. doi: 10.1074/jbc.274.34.23969. [DOI] [PubMed] [Google Scholar]
- Takahama M, Tsutsumi M, Tsujiuchi T, Nezu K, Kushibe K, Taniguchi S, Kotake Y, Konishi Y. Enhanced expression of Tie2, its ligand angiopoietin-1, vascular endothelial growth factor, and CD31 in human non-small cell lung carcinomas. Clin Cancer Res. 1999;5:2506–2510. [PubMed] [Google Scholar]
- Smith PJ, Teichert-Kuliszewska K, Monge JC, Steward DJ. Regulation of endothelin-B receptor mRNA expression in human endothelial cells by cytokines and growth factors. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S158–S160. doi: 10.1097/00005344-199800001-00045. [DOI] [PubMed] [Google Scholar]
- Potter CJ, Rurenchall GS, Xu T. Drosophila in cancer research: an expanding role. Trends Genet. 2000;16:33–39. doi: 10.1016/s0168-9525(99)01878-8. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]


