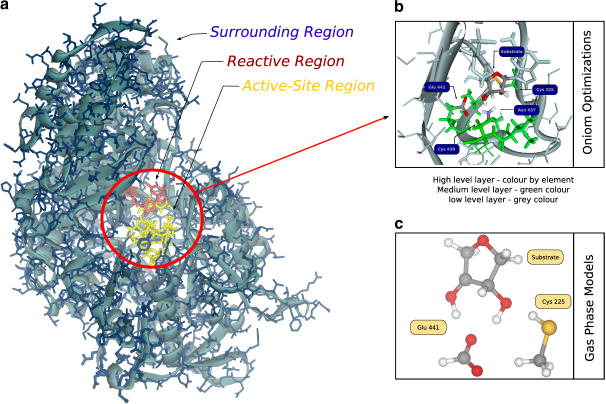
FIGURE 1.
(a) The E-S model obtained from the x-ray structure of the active site for R12 dimer of RNR with the bound substrate. The reactive region is colored red, the active site region is colored yellow and the surrounding region is colored blue. The blue background is indicative of a 10 Å layer of water molecules. (b) The division of the system in three theoretical levels for geometry optimizations. The high level layer is colored by element type, the medium level layer is colored green and the low level layer is colored blue. (c) The gas phase model. As geometry is optimized with the ribose ring and the active site residues disconnected from the protein, they can adopt the most favorable positions to the reaction without steric strain.