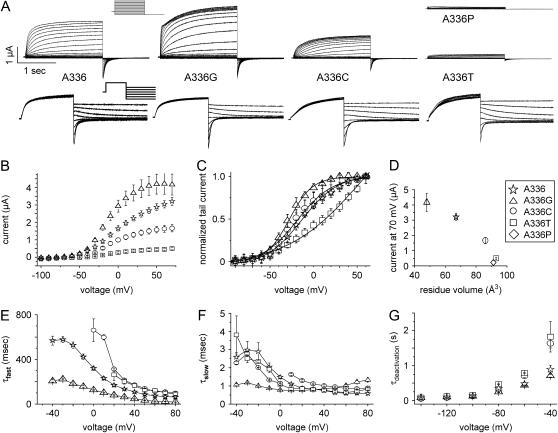
FIGURE 2.
Mutation of Ala-336 alters properties of KCNQ1 channel currents. (A) KCNQ1 currents recorded from Xenopus oocytes. Currents in the upper panel were elicited from a holding potential of −80 mV with pulses applied in 10 mV increments to potentials ranging from −120 to +80 mV. Currents in the lower panel were elicited from a holding potential of −80 mV with pulses to 40 mV and then deactivated by pulses from −140 to −40 mV applied in 20-mV increments. Lower traces were scaled to the same size to facilitate comparison. A-336P resulted in very small currents (∼0.2 μA at 40 mV), which could not be kinetically analyzed. (B) Current-voltage (I-V) relationship for WT and mutant channels. Currents were measured at the end of 2-s pulses and mean values ± SE are depicted (n = 11–13). (C) Voltage dependence of KCNQ1 channel activation determined by tail current analysis. The data were fit to the Boltzmann equation and values were KCNQ1 A-336G: V1/2 = −17.9 ± 0.6, k = 13.2 ± 0.5; KCNQ1 A-336: V1/2 = −3.7 ± 1–2, k = 21.1 ± 1.1; KCNQ1 A-336C: V1/2 = −8.8 ± 1.6, k = 20.4 ± 1.6, and KCNQ1 A-336T: V1/2 = 64.9 ± 13.2, k = 42.7 ± 4.8 (n = 11–13). (D) Currents measured at +70 mV are plotted versus the side-chain volume of the residues at KCNQ1 position 336 (n = 11–13). (E and F) Time constants for activation of KCNQ1 currents. The activating phase of currents was fit to two exponentials, and the fast and slow components were plotted versus voltage (n = 7–13). (G) Effect of mutation of residue Ala-336 on time constant of deactivation (τdeactivation) as a function of voltage (n = 3–13).