Abstract
Objective: There is a remarkable lack of scientific evidence to support the option to use alpha-stat or pH-stat management, as to which is more beneficial to brain protection during deep hypothermic CPB. This study examined cortical blood flow (CBF), cerebral oxygenation, and brain oxygen consumption in relation to deep hypothermic CPB with alpha-stat or pH-stat management. Methods: Twenty-two pigs were cooled with alpha-stat or pH-stat during CPB to 15 °C esophageal temperature. CBF and cerebral oxygenation were measured continuously with a laser flowmeter and near-infrared spectroscopy, respectively. Brain oxygen consumption was measured with standard laboratory techniques. Results: During CPB cooling, CBF was significantly decreased, about 52.2%±6.3% (P<0.01 vs 92.6%±6.5% of pH-stat) at 15 °C in alpha-stat, whereas there were no significant changes in CBF in pH-stat. While cooling down, brain oxygen extraction (OER) progressively decreased, about 9.5%±0.9% and 10.9%±1.5% at 15 °C in alpha-stat and pH-stat, respectively. At 31 °C the decreased value in pH-stat was lower than in alpha-stat (29.9%±2.7% vs 22.5%±1.9%; P<0.05). The ratio of CBF/OER were 2.0±0.3 in alpha-stat and pH-stat, respectively; it was kept in constant level in alpha-stat, and significantly increased by 19 °C to 15 °C in pH-stat (4.9±0.9 vs 2.3±0.4; P<0.01). In mild hypothermia, cerebral oxyhemoglobin and oxygen saturation in alpha-stat were greater than that in pH-stat (102.5%±1.4% vs 99.1%±0.7%; P<0.05). In deep hypothermia, brain oxygen saturation in pH-stat was greater than that in alpha-stat (99.2%±1.0% vs 93.8%±1.0%; P<0.01), and deoxyhemoglobin in pH-stat decreased more greatly than that in alpha-stat (28.7%±6.8% vs 54.1%±4.7%; P<0.05). Conclusions: In mild hypothermic CPB, brain tissue oxygen saturation was greater in alpha-stat than in pH-stat. However, cerebral oxygenation and brain tissue oxygen saturation were better in pH-stat than in alpha-stat during profound hypothermia. PH-stat strategy provided much more oxygen to brain tissue before deep hypothermic circulatory arrest.
Keywords: PH management, Cerebral oxygenation, Deep hypothermia, Cardiopulmonary bypass
INTRODUCTION
Neuropsychological deficits are still major causes of mortality and morbidity after cardiac operations. Although hypothermia reduces the metabolic rate and oxygen demand, and thus provides cerebral protection, the neurological complications of cardiopulmonary bypass (CPB) are significant with the neuropsychological impairment in 50% (Mora et al., 1996) and stroke in 2.5%, of patients (Gardner et al., 1985). The optimal pH strategy during CPB with deep hypothermic circulatory arrest remains controversial. The alpha-stat strategy continues to be widely used for pediatric and adult cardiac surgery though there are no good data to support this practice. A recently study demonstrated improved neurological outcome with pH-stat (Priestley et al., 2001). These findings were also confirmed in infants who underwent profound hypothermic bypass with or without circulatory arrest (du Plessis et al., 1997).
Clearly the optimal pH strategy during CPB with deep hypothermia requires further study. In the present study cerebral blood flow during profound hypothermic CPB with alpha-stat or pH-stat managements was monitored by laser Doppler flowmetry, a standard technique for flow measurements. Cerebral oxygenation was monitored using an experimental technique, near-infrared (NIR) spectroscopy. The combination of NIR spectroscopy, Laser Doppler Flowmetry, and standard laboratory techniques can provide information on the effects of different pH strategies on cerebral oxygenation, and changes in brain oxygen consumption during profound hypothermic CPB. The aim of this study was to determine whether pH-stat management has beneficial effect on brain oxygenation relative to the alpha-stat strategy in adult pig model.
Surgical preparation
Twenty-two adult pigs aged less than 5 months and weighing 56 to 73 kg were used after an acclimatization period of at least 12 days in the animal facility; feeding was withheld but water was given for 12 hours before surgery. Preanesthesia was induced with midazolam (0.3 mg/kg IM), ketamine (20 mg/kg IM), and atropine (0.02 mg/kg IM). After endotracheal intubation, the pig was ventilated mechanically with 60% oxygen and 40% air. The ventilator rate and tidal volume were adjusted to maintain arterial CO2 tension of 35 to 45 mmHg. Anesthesia was maintained with 1.5% to 2.0% isoflurance. Muscular paralysis was maintained with pancuronum 0.1 mg/kg given intravenously. A temperature probe was placed in the esophagus to monitor core temperature. Urine output was collected through a bladder catheter.
The right temporal muscle was partly excised. Two small holes (0.5 cm, 0.3 cm in diameter respectively) were made in the skull bone using a burr drill. The dura was exposed and intact. These holes were prepared for NIR spectroscopy and laser flowmeter probes. A median sternotomy was done by saw. The heart and cervical vessels were exposed. One small cannula was placed in the brachiocephalic artery through the right internal mammary artery for measuring the blood pressure and taking the blood samples during bypass. Another small cannula was placed in the right internal jugular vein beyond the venous valves via left internal mammary vein for taking venous blood samples. After treatment with Heparin (500 IU/kg), the CPB circuit was set up with the ascending aortic cannulation (22F) and a single atrium-cava tube (32 F) via the right atrium. The lungs were not inflated during bypass.
The CPB circuit consisted of Cobe roller pumps (model C22.2, Cobe, Arvada, CO), cardiotomy reservoir (Cobe HVRF 3700), arterial filter (40 micro, dideco D 733, Mirandola, Italy), water bath (Lauda MGW type RMSG, Postfach, Germany), and membrane oxygenator (Cobe Optima) with integrated heat exchanger. The circuit was primed with 1000 ml lactated Ringer’s solution, 500 ml Pentaspan, 25 ml of 1 mol/L sodium bicarbonate, and 5000 IU heparin. Sodium bicarbonate was administered as needed to maintain arterial blood pH within the normal range of 7.35 to 7.45. Steady CPB pump flow of 65–80 ml/(kg·min) was initially established, keeping mean BP>60 mmHg, a hematocrit of 20% during the bypass period. No intervention was made to control blood pressure during this study. Mean arterial blood pressure, central venous pressure, pump flow, and temperatures were followed continuously.
Experimental groups and protocol
Twenty-two pigs were randomly assigned to one of the following two groups. The alpha-stat animals (N=11) were maintained with an arterial carbon dioxide tension of 35–40 mmHg uncorrected for body temperature during deep hypothermic CPB. The pH-stat animals (N=11) received supplemental 5% carbon dioxide in the oxygenator gas mixture to maintain the temperature-corrected arterial carbon dioxide tension in the same range.
The pig was initially perfused for 15 min at normothermia (37 °C esophageal temperature) to stabilize body temperature, and check pH and gas results. Then, the CPB was cooled to an esophageal temperature of 15 °C with a temperature gradient maintained at less than 10 °C between the water bath and blood. It took about 30 to 35 min to cool down to 15 °C. The results of rCBF and NIR spectra were used for calculating the mean value for baseline from acquired three samples during 15 min CPB at 37 °C. Blood gases samples were taken every six-degree temperature down. Arterial and venous (jugular bulb) blood samples were obtained at fixed intervals: (1) 37 °C at 15 mins CPB, (2) 31 °C cooling, (3) 25 °C cooling, (4) 19 °C cooling, (5) 15 °C cooling.
Measurements
Cerebral cortical blood flow: Regional cerebral blood flow (rCBF) was continuously measured using an ALF 21R (Advance Company LdD, Tokyo, Japan) laser Doppler flowmeter fitted with a needle-type probe (Type Nspi: 9051 U). The data were recorded in absolute blood flow units once the reading was stable.
Cortical tissue oxygenation: Cerebral oxygenation was monitored using NIR spectroscopy (6500 NIR).
Brain oxygen consumption: Arterial and venous blood samples were obtained simultaneously at each stage of the protocol to monitor blood gases, pH and electrolytes. Blood gases were measured immediately after sample collection using a blood gas analyzer (Stat 9, NOVA Biomedical, Waltham, MA). Alpha-stat and pH-stat were used to manage blood pH. Oxygen content was calculated based on the formula: O2 content (vol%)=[HB]×1.36×SO2+PO2×0.003 (HB=hemoglobin concentration, SO2=oxygen saturation). Oxygen extraction was calculated using the formula: oxygen extraction (%)=(inflow O2 content−outflow O2 content)/inflow O2 content×100%.
Cerebral cortical blood flow/brain oxygen consumption ratio: It was calculated by the formula: rCBF:OER ratio=cerebral cortical blood flow/oxygen extraction.
Statistical analysis
All data are presented as mean±standard error of the mean (SEM). Mean cerebral blood flow, oxyhemoglobin, deoxyhemoglobin obtained during initial normothermic CPB were used as baseline levels and set at 100%. Statistical analysis was performed using the Statistical Analysis System (SAS Institute, Cary, NC). A repeated-measures ANOVA and Duncan’s multiple range test were used for comparison between different time points within a group, and Students’ t test was used for comparison between the two groups. A P value of less than 0.05 was considered significant.
RESULTS
Animal characteristics
The mean weight of the pigs was 60.5±0.8 kg and 60.7±0.9 kg in the alpha-stat and the pH-stat groups, respectively. Table 1 shows that hemodynamics were stable during the entire protocol in both groups. There were no differences between the two groups in blood pressure or pump flow during CPB. Hemoglobin levels in both groups decreased slightly due to fluid retention in the body requiring the use of additional crystalloid solution during cooling. There were no significant differences in the changes in hemoglobin between the two groups. Blood pH was maintained within normal ranges in both groups. The CPB perfusate temperature was similar in alpha-stat and pH-stat groups.
Table 1.
Blood gases, hematocrit, mean arterial pressure, and pump flow
| Variable Group | 37 °C CPB | 31 °C CPB | 25 °C CPB | 19 °C CPB | 15 °C CPB |
| PH | |||||
| Alpha-stat | 7.42±0.01 | 7.35±0.01 | 7.35±0.02 | 7.41±0.01 | 7.45±0.01 |
| PH-stat | 7.39±0.02 | 7.31±0.02 | 7.28±0.02 | 7.26±0.03* | 7.13±0.04* |
| PaCO2 (mmHg) | |||||
| Alpha-stat | 42.3±1.68 | 47.2±1.35 | 45.8±1.49 | 38.5±1.78 | 34.0±1.45 |
| PH-stat | 46.3±2.15 | 49.9±2.29 | 46.6±2.28 | 63.1±5.20* | 84.1±8.21* |
| PaO2 (mmHg) | |||||
| Alpha-stat | 396±24 | 411±26 | 416±25 | 421±27 | 428±30 |
| PH-stat | 394±22 | 408±24 | 411±25 | 419±26 | 422±28 |
| Hematocrit (%) | |||||
| Alpha-stat | 24.1±1.00 | 22.9±0.94 | 21.1±0.74 | 19.5±0.64 | 19.4±0.62 |
| PH-stat | 23.9±1.12 | 22.7±1.10 | 20.9±0.85 | 19.4±0.73 | 19.2±0.78 |
| Mean BP (mmHg) | |||||
| Alpha-stat | 63.6±2.14 | 58.7±2.11 | 59.1±1.86 | 61.2±1.78 | 64.5±1.82 |
| PH-stat | 62.6±1.50 | 60.0±1.39 | 59.1±0.68 | 60.6±1.05 | 60.2±0.17 |
| Pump flow [ml/(kg·min)] | |||||
| Alpha-stat | 68.9±3.07 | 68.1±2.97 | 69.3±2.63 | 68.1±2.79 | 67.3±2.68 |
| PH-stat | 70.2±3.45 | 69.5±3.26 | 70.8±3.47 | 68.9±3.12 | 67.8±2.98 |
Values are mean±SEM
P<0.05 vs alpha-stat
CPB=cardiopulmonary bypass; Alpha-stat=alpha-stat group; pH-stat=pH-stat group
Regional cerebral blood flow
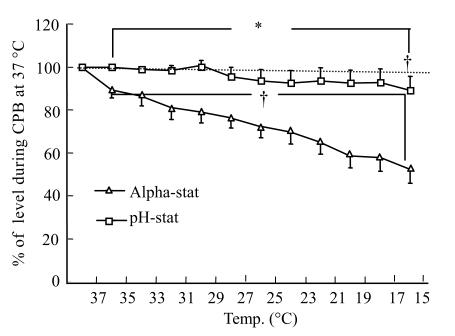
The mean value obtained during normothermic CPB was initially used as the baseline (100%). The rCBF response to CPB cooling was significantly different in the alpha-stat and pH-stat groups (Fig.1). In the alpha-stat group, rCBF at 15 °C decreased steadily to 52.2%±6.3% of the baseline level. Whereas, in the pH-stat group, there were no significant changes in the cerebral blood flow from CPB at 37 °C to 17 °C, but there was significant statistical difference during CPB at 15 °C (88.9%±6.6%) (P<0.05); which indicated a significant effect of temperature on rCBF during hypothermic CPB in alpha-stat protocol.
Fig. 1.
Change in brain tissue blood flow determined by laser flowmetry during hypothermia in both the alpha-stat and pH-stat. Levels obtained during initial cardiopulmonary bypass (CPB) at 37 °C were used as baseline
* P<0.05 vs alpha-stat; †P<0.05 vs baseline within the group
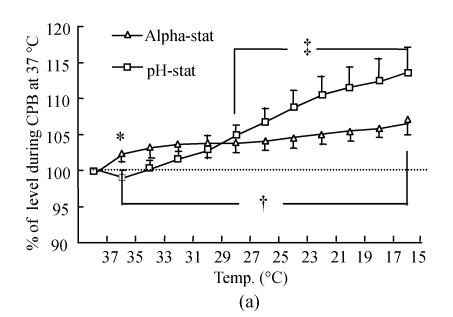
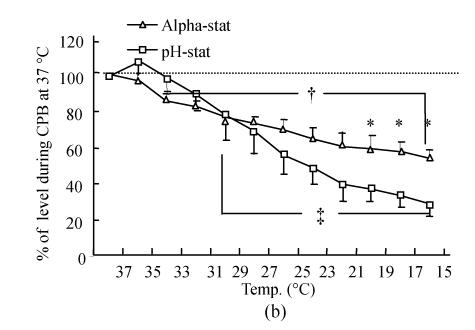
Tissue oxygenation
Oxyhemoglobin and deoxyhemoglobin were expressed as the ratio of oxyhemoglobin/total hemoglobin and deoxyhemoglobin/total hemoglobin. The level obtained during initial normothermic CPB was used as the baseline level (100%). The oxyhemoglobin level (Fig.2a) gradually increased when the esophageal temperature reached 15 °C in both groups. There were significant statistical differences in oxyhemoglobin during CPB at 27 °C to 15 °C compared with normothermic CPB within each group (P<0.01). There was no significant difference in oxyhemoglobin except at 35 °C (P<0.05) between the two groups. In contrast, the deoxyhemoglobin level (Fig.2b) progressively decreased during cooling, but the decreasing level at 15 °C expressed as a percent change from baseline in the pH-stat cooling groups (28.7%±6.8%) was greater than that in the alpha-stat cooling groups (54.1%±4.7%) (P<0.01).
Fig. 2.
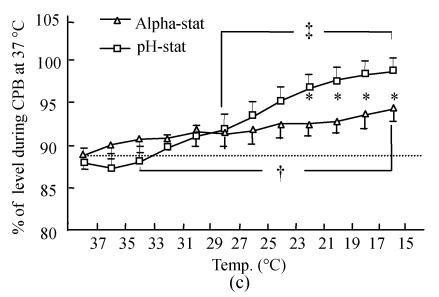
Changes in brain tissue oxyhemoglobin (a) and deoxyhemoglobin (b), as well as brain tissue oxygen saturation (c) determined by NIR spectroscopy during deep hypothermic CPB in both the alpha-stat and pH-stat. In (a) and (b), the levels obtained during initial CPB at 37 °C were used as baselines. The plus and double plus sign indicate P<0.05 vs levels obtained at 37 °C CPB within the group. *P<0.05 vs pH-stat
Tissue oxygen saturation was calculated using the formula: tissue oxygen saturation (%)=oxyhemoglobin/(oxyhemoglobin+deoxyhemoglobin)×100%. Tissue oxygen saturation (Fig.2c) during initial normothermic CPB was 88.7%±1.6% and 88.1%±1.7% in alpha-stat and pH-stat groups respectively. Following cooling down, oxygen saturation similarly increased; but the increasing level in the pH-stat groups (99.2%±1.0%) was greater than that in the alpha-stat groups (93.8%±1.0%). Existence of significant statistical differences in tissue oxygen saturation at 21 °C to 15 °C between the two groups (P<0.05) indicated that tissue oxygen saturation is greater in the pH-stat group was greater than that in the alpha-stat group during deep hypothermia.
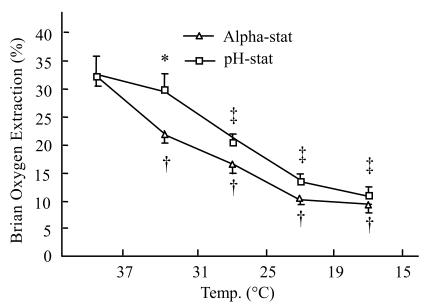
Brain oxygen extraction
Brain oxygen extraction during initial normothermic CPB was 32.3%±1.9% and 32.2±3.5% in alpha-stat and pH-stat groups, respectively. When cooling down, brain oxygen extraction progressively decreased, about to 9.5%±0.9% and 10.9%±1.5% in alpha-stat and pH-stat groups at 15 °C respectively. At 31 °C the decreasing level in the pH-stat group was lower than that in the alpha-stat group (29.9%±2.7% vs 22.5%±1.9%; P<0.05) (Fig.3).
Fig. 3.
Brain oxygen extraction during hypothermic CPB in both the alpha-stat and pH-stat. The plus and double pluses sign indicate P<0.05 vs levels obtained 37 °C CPB within the group. *P<0.05 vs alpha-stat
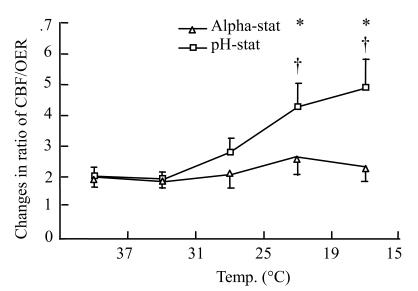
The ratio of cortical cerebral blood flow/brain oxygen consumption
The rCBF:OER ratios were initially 2.0±0.3 and 2.0±0.3 in alpha-stat and pH-stat groups, respectively. The ratio kept on the same level in alpha-stat group during hypothermia, but from 19 °C to 15 °C significantly increased in pH-stat group; and there were significant statistical differences within the group, compared with alpha-stat group (4.9±0.9 vs 2.3±0.4; P<0.01) (Fig.4).
Fig. 4.
Changes in ratio of cerebral blood flow/brain oxygen extraction during deep hypothermic CPB in both the alpha-stat and pH-stat. *P<0.05 vs alpha-stat; #P<0.05 vs CPB at 37 °C
DISCUSSION
Two distinct protocols for acid-base management are used in hypothermic cardiopulmonary bypass in clinical practice. Alpha-stat arterial blood measured at 37 °C, pH 7.40 had PCO2 of 40 mmHg, whereas the in vivo hypothermic blood is hypocapnic and alkalotic. In pH-stat, arterial pH and PCO2 are maintained at constant values during cooling, such that pH-stat in vivo hypothermic blood is pH 7.40, the PCO2 is 40 mmHg, whereas the blood measured at 37 °C is hypercapnic and acidotic. Until recently, there has been a remarkable lack of evidence in the literature of consistent physiological benefit provided by one protocol over the other.
In our study, decreased cerebral blood flow has been demonstrated following cooling down in the alpha-stat management, but no significant changes were observed in the pH-stat protocol. Cerebral blood flow at 15 °C was down 48% compared with 37 °C baseline in the alpha-stat protocol. The changes associated with downward temperature mainly depend on the cerebral metabolism. At very low hypothermic temperatures, severe temperature reductions may impair cerebral vascular relaxation, which results in a disruption of pressure-flow autoregulation and changes in cerebral perfusion (Civalero et al., 1962). Theoretically, the arterial PCO2 decreases about 4.5% each °C and pH increases about 0.015 units each °C following increased carbon dioxide solubility in aqueous solutions during hypothermic temperature in alpha-stat protocol. The pH-stat method, compared to alpha-stat method, results in much greater PaCO2 due to the addition of extra carbon dioxide to the blood. So the difference in rCBF between alpha-stat and pH-stat during hypothermic CPB reflects the carbon dioxide effect on cerebral blood flow, because it is a potent cerebrovasodilator.
The oxygen extraction ratio reflects the cerebral metabolic activity and global oxygen supply: demand ratio. The brain normally extracts approximately 25%–30% of the oxygen supplied. Cerebral blood flow and cerebral metabolic rate are not uniform throughout the brain, because 80% of cerebral blood flow is distributed to gray matter and 20% to white matter. Actually, the percentage may reflect global oxygen metabolism and represents an average level during different hypothermic times. Our experimental results showed that the changes in brain oxygen extraction during deep hypothermia were the same in both groups, although its value at 31 °C was significantly greater in pH-stat than in alpha-stat groups. It is not certain whether the alpha-stat or pH-stat strategy has to reduce the brain oxygen metabolism following cooling down; as the brain still consumes oxygen even at 15 °C. The CBF was greater in pH-stat, and the cerebral oxygen extraction ratio in pH-stat is believed to be higher than that in alpha-stat. The data described previously showed that with mild-moderate degrees of hypothermia, pressure-flow autoregulation is intact, whereas deep hypothermic temperatures of 15 °C~20 °C increase the ratio of CBF/CMRO2 and result in a loss of pressure-flow autoregulation in alpha-stat (Schell et al., 1993). However, under controlled cerebral perfusion pressure, the ratio of cerebral blood flow and brain oxygen extraction kept at a constant level in alpha-stat group in our results, but increased during 15 °C–19 °C CPB in pH-stat. These results suggested that in an adult pig, there is “flow-metabolism coupling” during deep hypothermic CPB in alpha-stat strategy; and that the coupling is lost and resulted in profuse brain blood flow in pH-stat management.
Near infrared spectrophotometry had been used as a noninvasive, real-time, online monitor to determine cerebral oxygenation state in humans and other animals (Lassnigg et al., 1999; Kadoi et al., 1999). In our study, the initial value of cortical O2 sat at 37 °C CPB was 88.7%±5.2% and 88.1%±5.3% in alpha-stat and pH-stat groups, respectively. These values are reasonable because NIRS views the entire vascular bed (arterial, capillary, venous) in an NIR spectrum. However, more than 80% of cerebral blood volume is capillary and venous blood, and the probability of a photon’s emerging from a large vein or artery is small. Actually, these data that were measured with NIRS at 37 °C were a little bit higher than those in other reports (Undar et al., 2000). The reason probably was that we directly placed the optode on the dura, thus getting rid of the effects from blood flow of scalp and skull during the experiment. During mild hypothermic CPB, the oxyhemoglobin and oxygen saturation in the brain were greater in alpha-stat than in pH-stat management. This should explain the balance between brain blood flow and oxygen metabolism. Our finding that pH-stat management decreases oxyhemoglobin affinity and may enhance oxygen availability, was confirmed by the brain oxygen extraction ratio at 31 °C CPB in our experiment (29.9%±2.7%:22.4%±2.2%, pH-stat vs alpha-stat P<0.05). Mild hypothermic temperature slightly affected the CBF in alpha-stat management. Because the oxygen availability in the brain is lower, the oxyhemoglobin and oxygen saturation were relatively higher in the alpha-stat group. Following continued significant lowering of temperature, the CBF was considerably affected in alpha-stat strategy, and cerebral oxygen metabolic rate decreased equally in alpha-stat and pH-stat strategies. The cerebral oxyhemoglobin and oxygen saturation tended to be higher in pH-stat than in alpha-stat strategies during moderate hypothermic CPB. Cerebral oxygen saturation significantly increased and deoxyhemoglobin decreased in the pH-stat strategy during profound hypothermic CPB; which revealed that in pH-stat, the brain tissue owned more oxygen than that in alpha-stat.
Although cerebral blood flow and oxyhemoglobin and O2 sat were relatively high during deep hypothermia, these changes should not be considered as indications of “luxury perfusion”. Hypothermia decreases cerebral oxygen consumption; but also greatly increases the oxygen affinity of hemoglobin or results in alterations in tissue oxygen diffusion. As temperature decreases, increased hemoglobin oxygen affinity may impair oxygen transfer from hemoglobin to mitochondria of brain cell. Thus, oxygen availability at the tissue level was diminished. Cytochrome aa3 is the terminal oxidase which accepts oxygen at the end of the respiratory chain and is responsible for nearly 90% of oxygen consumption in humans (Chan and Li, 1990). In alpha-stat, deep hypothermia causes increase of oxygen affinity of hemoglobin, decrease of hemoglobin values and alkalotic pH, which affects the function of cytochrome aa3, leading possibly to impaired tissue oxygenation. PH-stat management during deep hypothermic CPB increases cortical blood flow and cerebral oxygen saturation and may increase tissue hemoglobin concentration and dissolved oxygen. It is believed that the higher the dissolved oxygen concentration in the pH-stat group relative to alpha-stat management is (because pH-stat management increases brain oxygen supply, and acidosis shifts the oxyhemoglobin curve to the right), the greater is the amount of brain tissue PO2 During deep hypothermia, the dissolved oxygen concentration contributes to the total tissue oxygen concentration, and the brain uses mostly dissolved oxygen (Dexter et al., 1997).
In conclusion, in an adult porcine model which underwent deep hypothermic CPB, cortical cerebral blood flow significantly decreased and the ratio of rCBF/OER remained at constant level following the cooling down of temperature in alpha-stat management, but there were no significant changes in cortical cerebral blood flow and increased rCBF/OER ratio in pH-stat management. In mild hypothermia, brain tissue oxygen saturation in alpha-stat was greater than that in pH-stat. Cerebral oxygenation and brain tissue oxygen saturation in the pH-stat strategy were better than those in alpha-stat in profound hypothermia and provide much more oxygen to brain tissue before deep hypothermic cardiac arrest.
References
- 1.Chan S, Li P. Cytochrome c oxidase: understanding nature’s design of a proton pump. Biochemistry. 1990;29:1–12. doi: 10.1021/bi00453a001. [DOI] [PubMed] [Google Scholar]
- 2.Civalero LA, Moreno JR, Senning A. Temperature conditions and oxygen consumption during deep hypothermia. Acta Chir Scand. 1962;123:179–188. [PubMed] [Google Scholar]
- 3.Dexter F, Kern FH, Hindman BJ, Greeley WJ. The brain uses mostly dissolved oxygen during profoundly hypothermic cardiopulmonary bypass. Ann Thorac Surg. 1997;63:1725–1729. doi: 10.1016/s0003-4975(97)00297-x. [DOI] [PubMed] [Google Scholar]
- 4.du Plessis AJ, Jonas RA, Wypij D, Hickey PR. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1000. doi: 10.1016/S0022-5223(97)70013-8. [DOI] [PubMed] [Google Scholar]
- 5.Gardner TJ, Horneffer PJ, Manolio TA, Pearson TA, Gott VL, Baungartner WA, Borkon AM, Watkins IJr, Reitx BA. Stroke following coronary artery bypass grafting: a ten-year study. Ann Thorac Surg. 1985;40:574–581. doi: 10.1016/s0003-4975(10)60352-9. [DOI] [PubMed] [Google Scholar]
- 6.Kadoi Y, Kawahara F, Saito S, Morta T, Kunimoto F, Goto F, Fujita N. Effects of hypothermic and normothermic cardiopulmonary bypass on brain oxygenation. Ann Thorac Surg. 1999;68:34–39. doi: 10.1016/s0003-4975(99)00306-9. [DOI] [PubMed] [Google Scholar]
- 7.Lassnigg A, Hiesmayr M, Keznickl P, Mullner T, Ehrlich M, Grubhofer G. Cerebral oxygenation during cardiopulmonary bypass measured by near-infrared spectroscopy: Effects of hemodilution, temperature, and flow. J Cardiothorac Vasc Anesth. 1999;13:544–548. doi: 10.1016/s1053-0770(99)90005-8. [DOI] [PubMed] [Google Scholar]
- 8.Mora CT, Henson MB, Weintraub WS, Murkin JM, Martin TD, Craver JM, Gott JP, Guyton RA. The effect of temperature management during cardiopulmonary bypass on neurologic and neuropsychological outcomes in patients undergoing coronary revascularization. J Thorac Cardiovac Surg. 1996;112:514–522. doi: 10.1016/S0022-5223(96)70280-5. [DOI] [PubMed] [Google Scholar]
- 9.Priestley MA, Golden JA, O’Hara IB, McCann J, Kurth CD. Comparison of neurologic outcome after deep hypothermic circulatory arrest with alpha stat and pH stat cardiopulmonary bypass in newborn pigs. J Thorac Cardiovasc Surg. 2001;121:336–343. doi: 10.1067/mtc.2001.112338. [DOI] [PubMed] [Google Scholar]
- 10.Schell RM, Kern FH, Greeley WJ, Schulman SR, Frasco PE, Croughwell ND, Newman M, Reves JG. Cerebral blood flow and metabolism during cardiopulmonary bypass. Anesth Analg. 1993;76:849–865. doi: 10.1213/00000539-199304000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Undar A, Eichstaedt HC, Fraxier OH, Fraser CDJr. Monitoring regional cerebral oxygen saturation using near-infrared spectroscopy during pulsatile hypothermic cardiopulmonary bypass in a neonatal piglet model. ASAIO J. 2000;46:103–106. doi: 10.1097/00002480-200001000-00024. [DOI] [PubMed] [Google Scholar]