Fig. 6. Cltx inhibits glioma Cl− currents by inducing endocytosis of channels into caveoli.
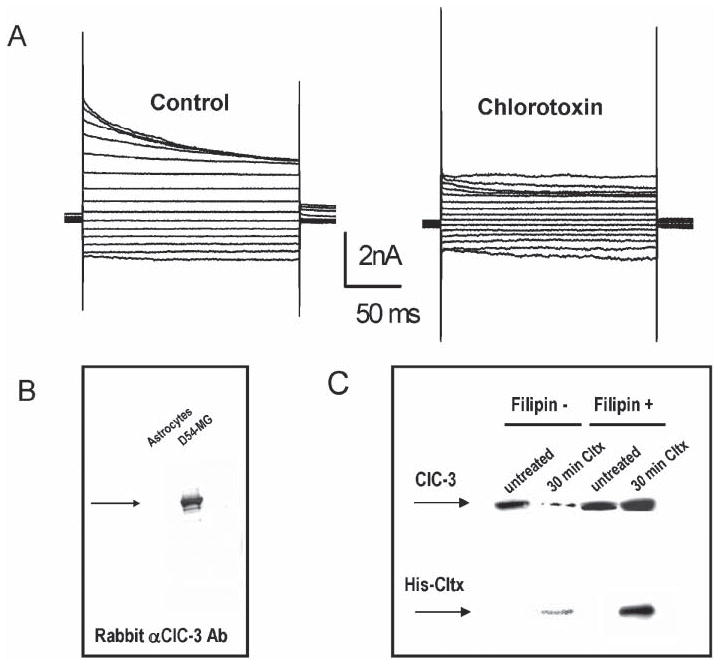
(A) Outwardly rectifying, inactivating Cl− currents recorded by whole-cell patch clamp recording in D54-MG glioma cells using voltage steps ranging from −120−160 mV (20 mV increments). Application of Cltx causes a slow and irreversible reduction of measurable currents. The example illustrated was recorded after 15 minutes. (B) Recombinant 6×-His-Cltx peptide was used to affinity purify interacting proteins from glioma lysates. The affinity-purified fraction was run on SDS-PAGE and probed with antibodies to ClC-3 to detect the channel in lysates from glioma cells but not from astrocytes. (C) A surface biotinylation approach, previously described by Ye et al. (1999) was used to assess the surface expression of ClC-3 protein. Separation over avidin beads isolated the cell-surface proteins that were accessible to the biotinylation reagent at the time of exposure. This fraction was probed by Western blot with antibodies to ClC-3 and anti-His to detect bound His-Cltx. ClC-3 protein was present in the membrane of untreated glioma cells. Treatment with Cltx for 30 minutes before exposure to the biotinylation reagent reduced the amount of ClC-3 on the membrane surface. Disrupting caveolar endocytosis with 5 μg ml−1 Filipin (a sterol-binding drug) prevents Cltx-mediated reduction in surface expression of ClC-3 in these cells, which indicates that Cltx causes endocytosis of ClC-3 into caveoli.
