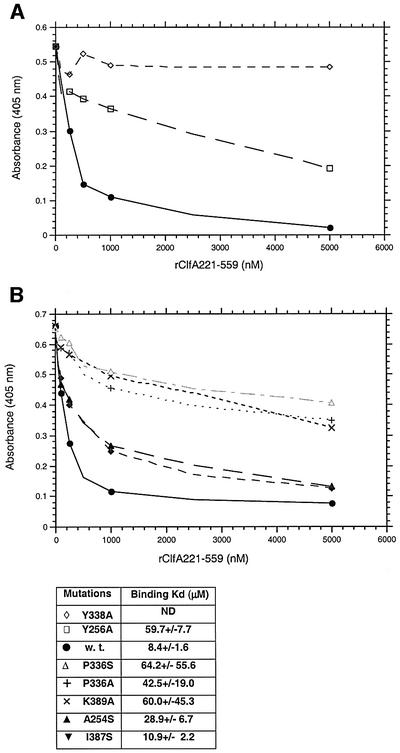
Fig. 7. (A) The relative affinities of rClfA221–559w.t. (closed circles), rClfA221–559Y256→A (open squares) and rClfA221–559Y338→A (open diamonds) for Fg were examined in an inhibition ELISA-type assay (Smith et al., 1993). Biotin-labeled rClfA221–559 was incubated with the indicated increasing concentration of unlabeled ClfA proteins in Fg-coated microtiter wells. The amount of labeled ClfA protein bound to the immobilized Fg was then quantitated. The Kd of each protein was calculated by determining the affinity of the proteins for a fluorescein-labeled 17-amino-acid-long synthetic peptide representing the C-terminus of the Fg γ-chain using fluorescence polarization. (B) The relative affinities of rClfA221–559A254→S (upright closed triangles), rClfA221–559I387→S (inverted closed triangles), rClfA221–559 K389→A (×), rClfA221–559P336→A (+), rClfA221–559P336→S (open upright triangles) for Fg were examined in the same way as above. Kd was also calculated as indicated.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.