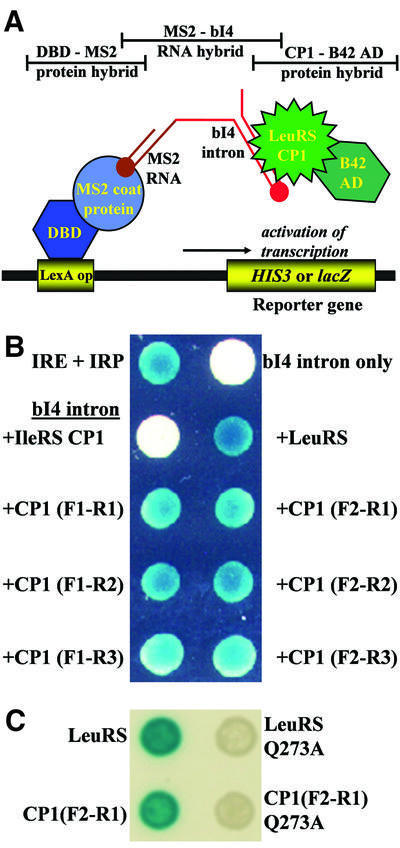
Fig. 3. Three-hybrid analysis detects binding interactions between the isolated LeuRS CP1 domains and the bI4 intron. (A) Cartoon of the three-hybrid model. The two protein hybrids are the DBD–MS2 coat protein fusion (shades of blue) and the LeuRS CP1 domain linked to B42 AD (shades of green). The bait RNA is comprised of MS2 RNA (brown) and the bI4 intron (red). Intracellular β-galactosidase reporter activity was detected by growing transformed yeast cells on solid agarose synthetic medium (Ura–, Trp–) which contained 2% galactose, 1% raffinose and 20 mg/ml X-gal (Rho and Martinis, 2000). (B) The bait bI4 intron RNA and prey proteins were expressed from the plasmids pRH3′ and pYESTrp2, respectively. Blue color developed for both the positive control (IRE and IRP) and each yeast colony that expressed bI4 intron as bait and either full-length or fragments of LeuRS that contained CP1 as prey. Expression of the bI4 intron alone (upper right corner) or with the IleRS CP1 domain (left, second from top) failed to yield blue colonies. (C) When the bait bI4 intron was tested the full-length (top right) or the smallest fragment (bottom right) of LeuRS that contained the Q273A mutation, a three-hybrid response was not detected.
