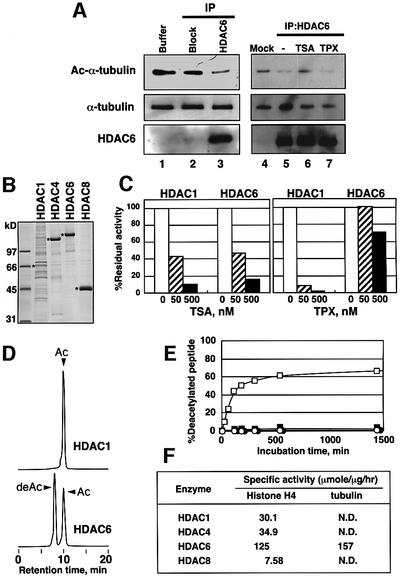
Fig. 3. In vitro deacetylation of α-tubulin by HDAC6. (A) Deacetylation of acetylated microtubules by HDAC6 from mouse testis. HDAC6 was isolated from mouse testis by immunoprecipitation using an anti-mouse HDAC6 antibody raised against an HDAC6 peptide (Verdel et al., 2000). As a control, the antigen peptide was added in excess to block the antibody binding to HDAC6 (Block). HDAC6 was incubated with acetylated microtubules isolated from TSA-treated cells in 20 µl for 3 h at 37°C in the presence or absence of TSA (100 nM) or TPX (100 nM). The success of the immunoprecipitation and the deacetylation of the microtubules were visualized by immunoblotting using the anti-mouse HDAC6 and anti-acetylated tubulin antibodies, respectively. (B) Purification of HDAC enzymes produced in insect cells via a baculovirus system. Asterisks denote the produced enzymes in the CBB stained gel of SDS–PAGE. HDAC4, HDAC6 and HDAC8 were efficiently expressed and highly purified. Although the production of HDAC1 was not efficient, the preparation had a sufficient activity to deacetylate 3H-histone. (C) Effects of TSA and TPX on the recombinant enzymes. The enzyme activities of HDAC1 and HDAC6 were determined with 3H-histone in the presence of various concentrations of TSA and TPX. (D) In vitro deacetylation of an acetylated tubulin peptide by recombinant human HDAC6. Recombinant human HDAC6 was incubated for 3 h with 0.5 mM Dns-tubulin peptide containing acetylated Lys40, and the reaction mixtures were analyzed with HPLC using a fluorescent detector. The retention time of the new peak (deAc) was identical to that of Dns-tubulin peptide without acetylation. (E) Enzyme specificity of deacetylation. The deacetylation of the Dns-acetylated tubulin peptide was analyzed over time with recombinant HDAC1, HDAC4, HDAC6 and HDAC8. The enzyme preparations used for the deacetylation assay were normalized based on their specific activities obtained with 3H-histone. The amounts of the deacetylated peptide after incubation with HDAC1 (open circles), HDAC4 (filled circles), HDAC6 (open squares) and HDAC8 (filled squares) for various lengths of time were plotted. (F) Specific activities of the recombinant enzymes for deacetylation of tubulin and histone H4 peptides. N.D., not detected.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.