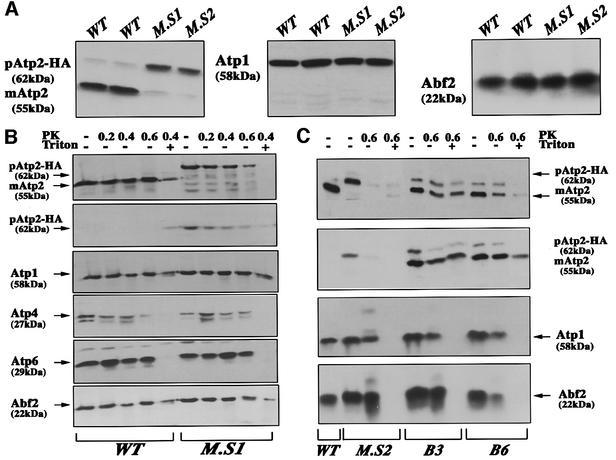
Fig. 4. Amount of Atp2 protein and localization in the ATP2-3′UTRADH1 strain. (A) Mitochondria were prepared from wild-type cells (WT) as from two independent clones of the ATP2-3′UTRADH1 strain (M.S1 and M.S2). SDS–PAGE was performed with 30 µg of proteins and analyzed by western blotting using antibodies against Atp2, Atp1 and Abf2 proteins.The precursor of Atp2p migrated at an approximate mol. wt of 62 kDa, while the mature protein migrated at ∼55 kDa. (B) To determine the precise cellular localization of Atp2p, mitochondria were treated for 30 min at 0°C with PK at 0.2, 0.4 or 0.6 mg/ml (PK); the PK digestion at 0.4 mg/ml was also performed in combination with 1% Triton X-100 (Triton). The following antibodies were used: polyclonal antibodies against Atp2p, Atp1p, Atp4p, Atp6p, Abf2p and monoclonal antibody 12CA5 against the HA epitope (second from the top). The precursor of Atp2p migrated at an approximate mol. wt of 62 kDa, while the mature protein migrated at ∼55 kDa. In wild-type mitochondria, no signal was detected with the 12CA5 antibody, thus confirming the identity of the revealed 62 kDa polypeptide with the protein expressed from the altered ATP2 allele. Moreover, the HA-tagged version of the Atp2 precursor was almost entirely digested by a 0.6 mg/ml concentration of PK, while the mature form of the protein in wild-type cells was more protected against digestion. These data indicate that Atp2 protein synthesized from the altered allele is present at the mitochondrial surface, but its mitochondrial import is not efficient. (C) To determine the cellular localization of a HA-tagged version of Atp2, mitochondria were purified from B3 and B6 cells and subjected to western blotting after digestion with 0.6 mg/ml PK in the presence or absence of 1% Triton X-100. As controls, CW04 strain (WT) and ATP2-3′UTRADH1 strain (MS2) were used. Antibodies against Atp2, Atp1 and Abf2 proteins and against the HA epitope (second from the top) were used successively. Even though we were able to detect more of the HA-tagged Atp2p precursor in B3 and B6 cells than in wild-type cells, the amount of the mature Atp2p insensitive to externally added protease in B3 and B6 cells was significantly increased as compared with that observed in the MS2 strain, thus confirming that the impairment of Atp2p mitochondrial import observed in the ATP2-3′UTRADH1 strain is not due to the presence of the three HA epitopes at the C-terminus of the protein.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.