Figure 2.
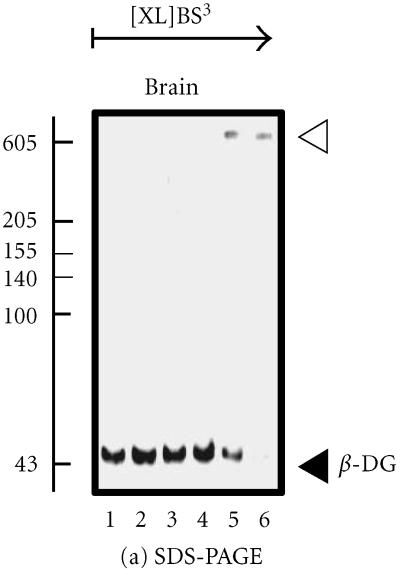
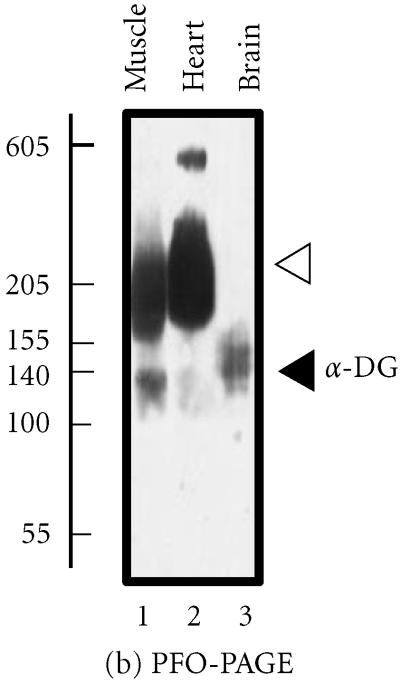
Analysis of the brain dystroglycan complex. Panel (a) shows the chemical cross-linking analysis of β-dystroglycan (β-DG) of apparent 43 kd in normal mouse brain using the hydrophilic probe bis-sulfosuccinimidyl-suberate (BS3) and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Detection of this sarcolemmal protein was achieved by immuno-decoration with monoclonal antibody NCL-b-DG [10]. Lanes 1–6 represent 0, 25, 50, 100, 200, and 300 μg BS3/mg protein, respectively. Increasing concentrations of crosslinker (XL) clearly stabilized a high-molecular-mass complex demonstrating the oligomeric status of brain β-dystroglycan. In panel (b), is shown the perfluoro-octanoic acid polyacrylamide gel electrophoresis (PFO-PAGE) [9] analysis of α-dystroglycan (α-DG). Lanes 1 to 3 represent mouse skeletal muscle, cardiac muscle, and brain microsomal membranes, respectively. The highly glycosylated, extracellular protein α-dystroglycan was immuno-decorated employing monoclonal antibody VIA12 [10] . Compared to skeletal and cardiac muscle, the dystroglycan sub-complex was not preserved in brain by this method, indicating a more tentative dystroglycan binding in the central nervous system. The position of monomers and oligomers is marked by closed and open arrow heads, respectively. The relative position of molecular mass markers (in kd) is indicated on the left of blots.