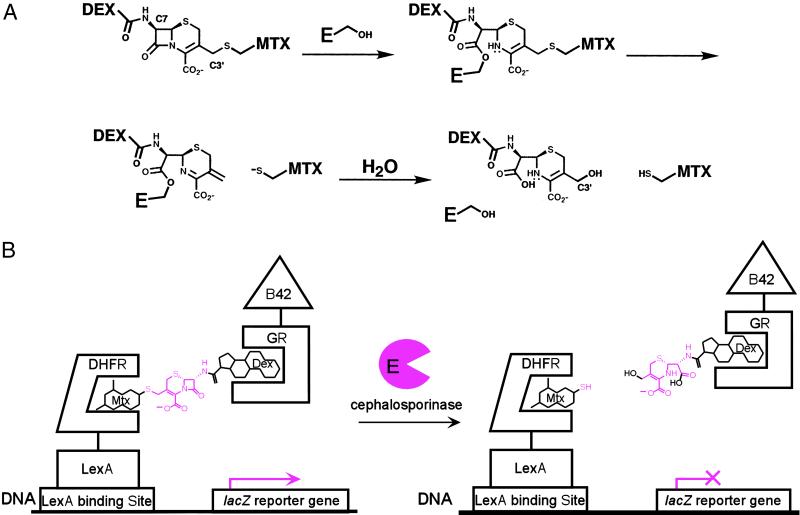
Fig 2.
Cephalosporinase model reaction. Cephalosporin hydrolysis provides a simple cleavage reaction to demonstrate the complementation strategy. (A) Cephalosporin hydrolysis by a cephalosporinase enzyme. Cephalosporinases are serine-protease enzymes and catalyze the hydrolysis of cephalosporin antibiotics by means of an acyl-enzyme intermediate. Hydrolysis of the β-lactam bond in Mtx-Cephem-Dex results in expulsion of the leaving group at the C3′ position of the cephem core, effectively breaking the bond between Mtx and Dex. (B) Cephalosporin hydrolysis by the cephalosporinase enzyme disrupts transcription of a lacZ reporter gene. The Mtx-Cephem-Dex substrate dimerizes a LexA DNA-binding domain-dihydrofolate reductase (LexA-DHFR) and a B42 activation domain-glucocorticoid receptor (B42-GR) fusion protein, activating transcription of a lacZ reporter gene. Addition of active cephalosporinase enzyme results in cleavage of the Mtx-Cephem-Dex substrate and disruption of lacZ transcription.