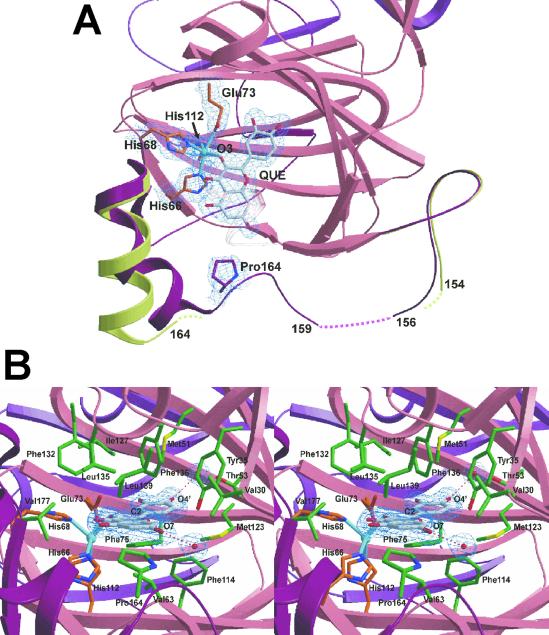
Fig. 2.
(A) Top view of the active site of 2,3QD⋅QUE, with a superposition of the linker regions of 2,3QD and 2,3QD⋅QUE. The N-terminal domain (residues 1–145), part of the C-terminal domain (206–350), and the linker region (146–205) of 2,3QD⋅QUE are represented in pink, violet, and dark red, respectively. The linker region of native 2,3QD is in light green. Pro164 and QUE make a van der Waals contact. This contact induces a conformational change in the linker region, stabilizing a large part of it. 2Fo-Fc electron density, for the copper site, bound quercetin, and Pro164, is contoured at the 1σ level. (B) View from the solvent into the active site of 2,3QD⋅QUE. Protein copper ligands are represented by orange sticks, QUE is in gray, and the residues in van der Waals contact with the substrate are in green. 2Fo-Fc electron density, for the substrate and the water molecules hydrogen bonded to it, is contoured at the 1σ level. Figs. 2 and 3 were generated with the program bobscript (41) and rendered with the program raster3d (42).