Abstract
Low levels of protein C (PC) predict outcome as early as 10 h after insult in a rat polymicrobial sepsis model and were associated with suppression of PC mRNA, upstream transcription factor FoxA2, and cofactor hepatocyte nuclear factor 6 (HNF6). Small interfering RNA suppression of FoxA2 in isolated hepatocytes demonstrated regulation of both its cofactor HNF6 and PC. Our data suggest that reduced FoxA2 may be important in the suppression of PC and resulting poor outcome in sepsis.
Severe sepsis, a devastating disorder with a mortality rate of 30 to 50% (1), occurs from a complex host response to insult following infection, which evolves into a cascade of inflammatory activation, microvascular coagulation, endothelial cell dysfunction, and ultimately organ failure and death (for a review, see reference 11). While many soluble factors change during sepsis, there is substantial evidence that a reduction in plasma level of the serine protease protein C (PC) is prognostic for sepsis and sepsis severity (for a review, see reference 10). Studies have suggested that PC deficiency may appear before the onset of defined clinical parameters of severe sepsis or septic shock (18). Moreover, a retrospective evaluation of the PROWESS clinical trial (4) indicated that severe PC deficiency was associated with early death resulting from refractory shock and multiple organ failure in sepsis (17). These studies support the important role of the PC pathway in response to infection, which is further exemplified by the efficacy of recombinant human activated PC (APC) in the treatment of severe sepsis (4).
PC, a member of the vitamin K-dependent family of blood coagulation proteins, is synthesized in the liver as an inactive zymogen. In settings of thrombotic stress, excess thrombin is generated and binds to endothelial surface thrombomodulin; this complex proteolytically generates APC. APC functions as a feedback inhibitor of thrombin generation by cleavage of coagulation factors Va and VIIIa. In addition, APC inhibits plasminogen activator inhibitor type 1 (8) and has receptor-mediated anti-inflammatory and apoptotic effects (14, 19, 23). Thus, APC plays a fundamental role in a coordinated system for controlling thrombosis, limiting inflammatory responses, and potentially decreasing endothelial cell apoptosis in response to inflammatory cytokines and ischemia (14).
The factors that control the reduction in PC during acute inflammatory response in sepsis are not fully understood. Based on the underlying microvascular coagulopathy observed with sepsis, the conventional explanation has been consumption of endogenous anticoagulants, e.g., PC and antithrombin III, due to the inflammatory insult and subsequent activation of the extrinsic coagulation cascade (13). Previously, we provided evidence for suppression of PC levels in a rat model of sepsis, although the mechanism was not elucidated (12). In this study, we have explored the mechanism for the suppression of PC in a rat cecal ligation and puncture (CLP) model of polymicrobial sepsis.
Details of the CLP model have been previously described (12). Briefly, female Sprague-Dawley rats (each, 245 to 265 g) were purchased from Harlan (Indianapolis, IN) and allowed to acclimate a minimum of 6 days prior to surgery. Rats were anesthetized with 3% isoflurane (1:1.5 with O2), and polyethylene catheters (Strategic Applications, Inc., Libertyville, IL) were implanted surgically into the femoral vein. Immediately following femoral catheterization, CLP was performed with a single puncture with a 16-gauge needle to obtain an expected mortality of ∼75%; care was taken to ligate the same length of cecum (1 cm as measured by a ruler on the scalpel). Following surgery, the rats were given ketoprofen (2 mg/kg of body weight) intramuscularly for pain relief, injected subcutaneously with 5 ml of prewarmed saline and then continuously infused with 5% dextrose in 0.9% saline (Abbott Laboratories, North Chicago, IL) at a rate of 2 ml/h via the femoral catheter until death or at the endpoint of the study. Sham treatment rats received identical surgery (except for CLP) and postoperative management. An enzyme-linked immunosorbent assay (ELISA) for measurement of PC levels was performed as described previously (12), and purified recombinant rat PC was used as a reference standard. All experimental methods were approved by the institutional animal care and use committee and were in accordance with the institutional guidelines for the care and use of laboratory animals. One-way analysis of variance or analysis of covariance was used to determine statistical significance with JMP5.1 software (SAS Institute). Data are presented as means ± the standard error (SE), unless indicated otherwise. A P value of <0.05 was considered significant.
PC expression is suppressed in a rat model of sepsis.
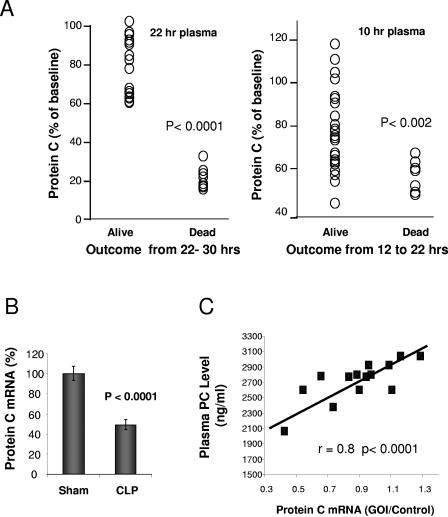
As shown in Fig. 1A, analysis of plasma PC levels 22 h post-CLP showed that all animals that subsequently died within the first 30 h had significantly lower plasma PC levels, which were predictive of early death. Animals that died after 30 h or survived until the end of the study (96 h) did not have significantly reduced PC levels, as previously shown (12). Receiver operator characteristic (ROC) curves, generated from logistic regression models (JMP5.1 software), showed that a cutoff value of 60% baseline PC level predicted death by 30 h with a sensitivity and specificity of 100%. During the course of these studies, we noted that some animals died even before the 22-h time point, so we performed studies to assess the impact of low PC at an earlier time point of 10 h post-CLP on mortality from 10 to 22 h. As shown, even at this earlier time point, animals that died between 10 and 22 h all had low PC levels, which were significantly lower than those from the mean number of animals still alive at the 22-h time point. ROC analysis of death from 10 to 22 h predicted outcome with a sensitivity of 88% and a specificity of 84% (area under the ROC curve of 0.88).
FIG. 1.
Expression of protein C in rat CLP. (A) Analysis of protein C plasma levels at 10 and 22 h as a function of subsequent survival in a rat CLP model of sepsis. Blood draws were from the retro-orbital sinus, and the plasma was analyzed for PC concentrations by ELISA. Early deaths were examined for animals that died within either the 10- to 22-h or the 22- to 30-h time frames. (B) Effect of induction of sepsis by CLP on liver protein C mRNA expression. cDNA was synthesized by using total RNA isolated from the livers of control-sham and CLP animals sacrificed after final blood samplings. For determination of relative mRNA levels, the cDNA was analyzed by qPCR (TaqMan). An internal standard curve was generated by serial dilution of an appropriate cDNA reaction and used for relative quantification. The assay for 18S rRNA was the Eukaryotic Endogenous Control kit (ABI, Foster City, CA) and was used to normalize to the gene of interest (GOI). Results are from four independent experiments with 42 animals (20 sham and 22 CLP). (C) Relationship of liver PC mRNA to plasma PC concentration by ELISA at 22 h post-CLP.
In separate experiments, animals were sacrificed at the 22-h time point for collection of liver tissue, and total RNA was purified using RNeasy (QIAGEN) and analyzed by quantitative real-time PCR (qPCR) with an ABI Prism 7900HT Sequence Detection system. CLP animals sacrificed at 22 h post-CLP showed a significant reduction in liver PC mRNA levels compared to surgical sham animals (Fig. 1B). PC liver mRNA levels were consistently repressed to 52 to 67% of the sham level in four independent CLP studies. An examination of individual animals in the CLP group showed a high correlation between the levels of protein C mRNA and plasma PC (Fig. 1C).
Transcription factor FoxA2 is suppressed in the CLP model.
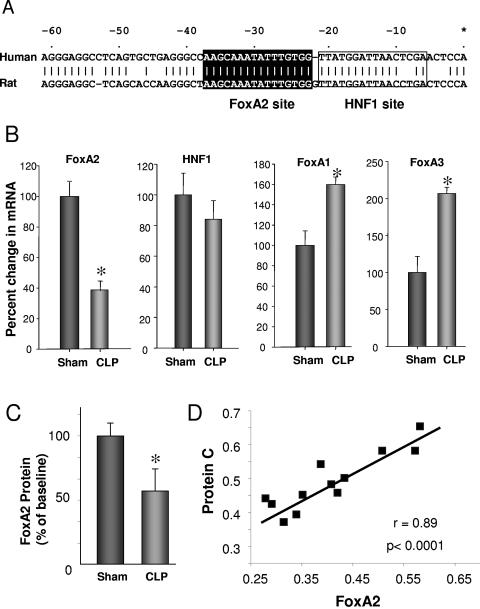
Previous studies by Tsay et al. and other researchers (26, 27, 29, 30) identified regions of the PC promoter important for liver-specific expression of PC and cis elements corresponding to hepatocyte nuclear factor 1 (HNF1), HNF3 (FoxA), and NF-I/CTF binding sites sufficient for basal promoter activity. However, the factors that control PC expression in vivo, especially under conditions of inflammatory stress, have not been elucidated. Shown in Fig. 2A are the upstream regulatory regions for the human and rat protein C promoters. There is a significant conservation of sequence in this region, including identical sites for the forkhead transcription factor FoxA2 and near-identity to HNF1 binding sites. We examined whether there were any differences in the levels of these transcription factors in the liver following CLP; as shown in Fig. 2B, FoxA2 expression was substantially reduced at 22 h post-CLP. In contrast, levels of the related family members FoxA1 and FoxA3 were actually increased following CLP, suggesting that the decrease of FoxA2 was not a general suppression of this family; HNF1 was not significantly changed relative to the surgical sham group. As shown in Fig. 2C, the level of FoxA2 protein was also found to be reduced by an immunoassay performed as described previously (7) using commercially available antibody sc-9187 against FoxA2 (Santa Cruz).
FIG. 2.
Comparison of the PC promoter region of the human and rat. (A) Analysis of the upstream region of the human and rat PC genes with MatInspector, which identified the noted FoxA2 (perfect match to the core; matrix score of 0.993) and HNF1 binding sites. The numbering is relative to the transcription start site of the human PC gene. (B) Change in expression of transcription factors FoxA1, -2, and -3 and HNF1 at 22 h after induction of sepsis by CLP determined by qPCR (TaqMan). Eight animals per group were used for determining Fox factors, 13 animals were used for sham conditions, and 14 animals were used for CLP for HNF1 analysis. *, P < 0.05. (C) Inhibition of FoxA2 by immunoassay following CLP. *, P < 0.05. (D) Relationship of levels of FoxA2 and PC mRNA at 22 h after induction of sepsis by CLP. Values are the ratio of the GOI over the 18S control as described in the legend to Fig. 1.
The observations of reduced FoxA2 expression in CLP animals suggested that the observed reduction in PC expression in the CLP model may be related to the reduction in FoxA2 expression. If so, one would expect a correlation between liver FoxA2 and PC mRNA levels. As shown in Fig. 2D, we in fact observed a strong positive relationship, i.e., animals with suppressed FoxA2 also had suppressed PC mRNA levels (and low plasma PC levels; data not shown). In contrast, there was no correlation between PC expression in the liver and HNF1 or either FoxA1 or FoxA3 expression.
FoxA2 and PC regulation.
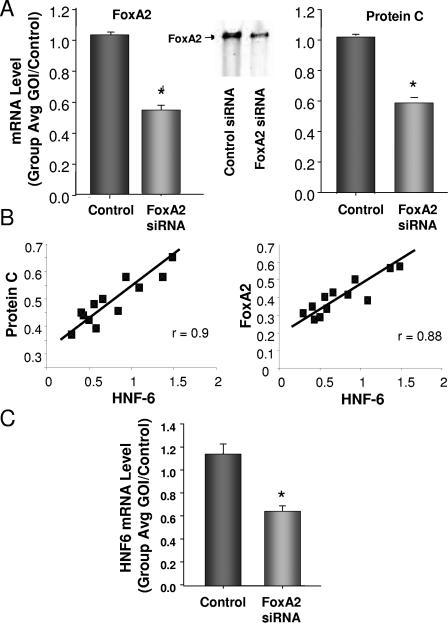
While the correlation with FoxA2 was highly suggestive of a direct relationship, to confirm that FoxA2 in fact could regulate the rat liver PC gene, we inhibited FoxA2 expression in isolated rat hepatocytes with a small interfering RNA (siRNA) and examined the effect of FoxA2 inhibition on PC expression. Freshly prepared rat hepatocytes were transfected with the siRNA using Lipofectamine 2000 (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer's protocol. The transfected cells were allowed to incubate at 37°C and 5% CO2 for 48 h. Two days posttransfection, RNA was prepared using RNeasy (QIAGEN, Inc., Valencia, CA) according to the manufacturer's protocol. To assess the level of FoxA2 binding to the PC promoter, we performed electrophoretic mobility shift assays as previously described (3) with detection using fluorescence-labeled oligonucleotides to the FoxA2 site (Fig. 2) obtained from LI-COR Biosciences (Lincoln, Nebr.) and 5′ end labeled with IRDye 800 phosphoramidite. As shown in Fig. 3A, the siRNA inhibited FoxA2 expression by approximately 50% at 48 h, significantly decreased the level of binding to the PC promoter, and resulted in a similar reduction in PC mRNA. A set of control siRNAs (Darmacon pool; D-001206) had no effect on either FoxA2 or PC mRNA.
FIG. 3.
Relationship of FoxA2 and PC expression. (A) Analysis of FoxA2 and PC expression levels and binding to the FoxA2 site in the PC promoter by electrophoretic mobility shift assay, following introduction of an siRNA for FoxA2 into rat hepatocytes (data are the results of four experiments). *, P < 0.01. Primary hepatocytes were obtained from Clonetics, Cambrex Bio Science Walkersville, Inc., Walkersville, MD. siGENOME SMARTpools for rat HNF3B (FoxA2), rat ONECUT1 (HNF6), and the nonspecific control pool were purchased from Dharmacon, Inc., Lafayette, CO. (B) Relationship between FoxA2, HNF6, and PC at 22 h after induction of sepsis by CLP. Values are the ratio of the GOI over the 18S control, as described in the legend to Fig. 1. (C) Analysis of the effect of an siRNA to FoxA2 on the expression of HNF6 in rat hepatocytes (data are the result of four experiments). *, P < 0.01.
Recent studies have shown that HNF6, a member of the onecut family of transcription factors, can modulate the activity of FoxA2 at its binding site (22). Therefore, we looked for any relationship between FoxA2 and PC expression with HNF6 expression in our CLP model. As shown in Fig. 3B, we observed a strong positive relationship between FoxA2 and HNF6 expression and with PC expression. These data suggested that following insult to the liver, FoxA2 suppression might be driving a decrease in HNF6 expression. We examined whether or not FoxA2 might directly modulate HNF6; as shown in Fig. 3C, we found that reduction in FoxA2 with an siRNA resulted in suppression of HNF6 mRNA expression in cultured hepatocytes. Thus, PC suppression appears to result from a primary reduction in the FoxA2 transcription factor, which subsequently results in a reduction of its cofactor HNF6.
FoxA2/HNF6 and outcome measures.
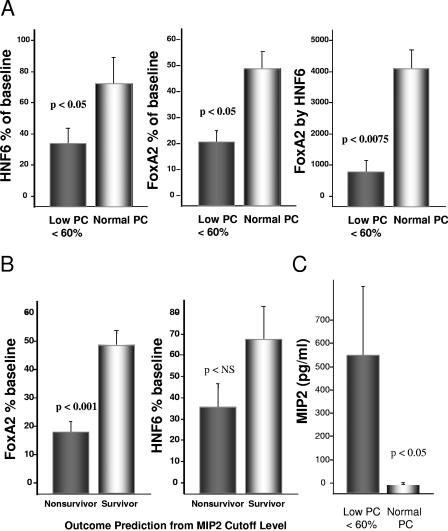
As it would not be feasible to determine liver FoxA2 levels in a survival study, we examined the level of both FoxA2 and HNF6 at 22 h as a function of a PC cutoff level of 60% of baseline as highly predictive of subsequent mortality, as determined above (Fig. 1A). As shown in Fig. 4A, animals with PC levels below the cutoff for early death had significantly reduced levels of both FoxA2 and HNF6. Moreover, an analysis of interaction between FoxA2 and HNF6 as a function of PC levels revealed an increased significance relative to each factor alone (P < 0.0075 versus P < 0.05). Consistent with the known interaction of HNF6 and FoxA2 noted above, these data suggest that during inflammatory insult PC suppression in the liver may be influenced more by the interaction of both factors than by each alone.
FIG. 4.
FoxA2, PC, and mortality predictors. (A) Relationship of HNF6 and FoxA2 to predictive plasma PC cutoff values for outcomes. Data are means ± SE for 14 animals. The cutoff value of 60% of the pre-CLP baseline was determined as described above by receiver operator characteristic curve analysis generated from the logistic regression model in the JMP5.1 software package. (B) Levels of FoxA2 and HNF6 expression in animals predicted to be survivors or nonsurvivors by MIP2 levels. Plasma samples were analyzed at baseline and 22 h post-CLP for MIP2 by ELISA. Data are means ± SE for 14 experiments. (C) Analysis of MIP2 plasma levels as a function of PC plasma levels. The measurement of MIP2 was by immunoassay, as previously described (12).
Previously, we found that the chemokines macrophage inflammatory protein 2 (MIP2) and KC/Gro were highly predictive of outcome in the rat sepsis model (12). Therefore, we used the predefined cutoff levels shown to predict mortality (specificity of 100% and 91% for MIP2 and KC/Gro, respectively, from ROC analysis); as shown in Fig. 4B, we observed that those animals predicted to be nonsurvivors, based on MIP2 levels, had significantly lower levels of FoxA2 expression. Identical results were found for KC/Gro (not shown), as levels of MIP2 and KC/Gro were highly correlated (r = 0.88; P < 0.0001). While we did not observe significance with HNF6 and predicted survival, there was a trend toward lower levels of HNF6 in predicted nonsurvivors. As a low PC level was predictive of outcome, we would expect animals below the PC cutoff of <60% to have high chemokine levels. As shown in Fig. 4C, this was in fact observed for MIP2 as well as KC/Gro (data not shown).
Because low FoxA2 expression correlated with high levels of these two CXC chemokines, we were interested in determining if there might be a direct effect on hepatocyte expression of FoxA2 or PC itself. However, treatment of hepatocytes with these two chemokines did not effect the expression of either (data not shown). We did observe a strong correlation of MIP2 levels with alanine aminotransferase levels (r = 0.80; P < 0.0001), suggesting that although not directly effecting FoxA2 expression, this chemokine may alter liver metabolism and, indirectly, FoxA2 and PC expression. As recently reviewed (25), the CXC chemokines including KC/Gro and MIP2 are increased in hepatic disorders, including ischemia, and play a role in neutrophil infiltration and injury. However, it is unclear whether these chemokines are simply markers of the degree of liver inflammation in our CLP model or have a more direct role in modulating FoxA2. While hepatic dysfunction is a significant component of the systemic inflammatory response (2), further studies are needed to elucidate the mediator(s) of liver FoxA2 suppression, following systemic inflammatory response and sepsis.
The incidence of sepsis is increasing due to multiple factors, including the aging population, increased immunocompromised patients, use of life-sustaining technology, and resistance to antimicrobial agents (1). There remains a significant need to understand the factors that predispose individuals to rapid conversion of an infection to sepsis, with poor resolution and death. PC appears to be such a factor, which predisposes to poor outcomes when suppressed. Recent studies have begun to elucidate the role of the protein C pathway in controlling normal physiology of the vasculature and of the innate immune system. Activated protein C modulates endothelial function by inhibiting cytokine signaling, suppressing cell adhesion and apoptosis, and promoting cell survival and angiogenesis (6, 11, 14-16, 19, 20, 23, 31). Moreover, APC appears to signal in monocytes, natural killer cells, (16), neutrophils (28), and eosinophils (9); it can directly inhibit the generation and release of cytokines and chemokines, including macrophage inflammatory protein 1α from THP-1 cells and human monocytes (5), and modulates macrophage migration inhibitory factor (24). Therefore, low endogenous PC levels during systemic inflammatory response may be pathophysiologically related to poor outcomes by reducing the ability to modulate both coagulopathic and inflammatory responses following infection.
Understanding the pathophysiology of systemic inflammatory response and sepsis is of significant importance in the ability to better diagnose and treat this disorder. Recent analyses have suggested that in clinical practice, there is significant difficulty in the diagnosis of sepsis, with frequent missed diagnoses (21). Our data may provide new understanding of early events in the response to infection that compromise host response and effect outcome and suggest that rapid inhibition of liver FoxA2 may be important in the early role of PC suppression in outcome determination. Moreover, our data open the opportunity of better understanding early targets that may be modulated to prevent PC suppression in critically ill patients.
Acknowledgments
We gratefully acknowledge Tonghai Zhang and Dianna Bailey for assistance with animal studies.
We are employees of Lilly Research Laboratories, a division of Eli Lilly and Co., which produces recombinant human activated protein C.
REFERENCES
- 1.Angus, D., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 2.Arias, I., W. Jacoby, H. Popper, et al. 1988. The liver: biology and pathobiology, 2nd ed. Raven Press, New York, N.Y.
- 3.Berg, D. T., L. J. Myers, M. A. Richardson, G. Sandusky, and B. W. Grinnell. 2005. Smad6s regulates plasminogen activator inhibitor-1 through a protein kinase C-beta-dependent up-regulation of transforming growth factor-beta. J. Biol. Chem. 280:14943-14947. [DOI] [PubMed] [Google Scholar]
- 4.Bernard, G., J. Vincent, P. Laterre, S. LaRosa, J. Dhainaut, A. Lopez-Rodriguez, J. Steingrub, G. Garber, J. Helterbrand, E. Ely, and C. Fisher. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-705. [DOI] [PubMed] [Google Scholar]
- 5.Brueckmann, M., U. Hoffmann, L. De Rossi, H. M. Weiler, V. Liebe, S. Lang, J. J. Kaden, M. Borggrefe, K. K. Haase, and G. Huhle. 2004. Activated protein C inhibits the release of macrophage inflammatory protein-1-alpha from THP-1 cells and from human monocytes. Cytokine 26:106-113. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, T., D. Liu, J. Griffin, J. Fernandez, F. Castellino, E. Rosen, K. Fukudome, and B. Zlokovic. 2003. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 9:338-342. [DOI] [PubMed] [Google Scholar]
- 7.Duong, D. T., M. E. Waltner-Law, R. Sears, L. Sealy, and D. K. Granner. 2002. Insulin inhibits hepatocellular glucose production by utilizing liver-enriched transcriptional inhibitory protein to disrupt the association of CREB-binding protein and RNA polymerase II with the phosphoenolpyruvate carboxykinase gene promoter. J. Biol. Chem. 277:32234-32242. [DOI] [PubMed] [Google Scholar]
- 8.Esmon, C. T., J. M. Gu, J. Xu, D. Qu, D. J. Stearns-Kurosawa, and S. Kurosawa. 1999. Regulation and functions of the protein C anticoagulant pathway. Haematologica 84:363-368. [PubMed] [Google Scholar]
- 9.Feistritzer, C., D. H. Sturn, N. C. Kaneider, A. Djanani, and C. J. Wiedermann. 2003. Endothelial protein C receptor-dependent inhibition of human eosinophil chemotaxis by protein C. J. Allergy Clin. Immunol. 112:375-381. [DOI] [PubMed] [Google Scholar]
- 10.Fisher, C. J., and S. B. Yan. 2000. Protein C levels as a prognostic indicator of outcome in sepsis and related diseases. Crit. Care Med. 28:S49-S56. [DOI] [PubMed] [Google Scholar]
- 11.Grinnell, B., and D. E. Joyce. 2001. Recombinant human activated protein C: a system modulator of vascular function for treatment of severe sepsis. Crit. Care Med. 29:S53-S61. [DOI] [PubMed] [Google Scholar]
- 12.Heuer, J. G., G. R. Sharma, B. Gerlitz, T. Zhang, D. L. Bailey, C. Ding, D. T. Berg, D. Perkins, E. J. Stephens, K. C. Holmes, R. L. Grubbs, K. A. Fynboe, Y. F. Chen, B. Grinnell, and J. A. Jakubowski. 2004. Evaluation of protein C and other biomarkers as predictors of mortality in a rat cecal ligation and puncture model of sepsis. Crit. Care Med. 32:1570-1578. [DOI] [PubMed] [Google Scholar]
- 13.Jacobi, J. 2002. Pathophysiology of sepsis. Am. J. Health Syst. Pharm. 59:S3-S8. [DOI] [PubMed] [Google Scholar]
- 14.Joyce, D. E., L. Gelbert, A. Ciaccia, B. Dehoff, and B. W. Grinnell. 2001. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J. Biol. Chem. 276:11199-11203. [DOI] [PubMed] [Google Scholar]
- 15.Joyce, D. E., and B. W. Grinnell. 2002. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-κB. Crit. Care Med. 30:S288-293. [DOI] [PubMed] [Google Scholar]
- 16.Joyce, D. E., D. R. Nelson, and B. W. Grinnell. 2004. Leukocyte and endothelial cell interactions in sepsis: relevance of the protein C pathway. Crit. Care Med. 32:S280-S286. [DOI] [PubMed] [Google Scholar]
- 17.Macias, W. L., and D. R. Nelson. 2004. Severe protein C deficiency predicts early death in severe sepsis. Crit. Care Med. 32:S223-S228. [DOI] [PubMed] [Google Scholar]
- 18.Mesters, R., H. Helterbrand, B. G. Utterback, S. B. Yan, B. Chao, and J. A. Fernandez. 2000. Prognostic value of protein C levels in neutropenic patients at high risk of severe septic complications. Crit. Care Med. 28:2209-2216. [DOI] [PubMed] [Google Scholar]
- 19.Mosnier, L. O., and J. H. Griffin. 2003. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease activated receptor-1 and endothelial cell protein C receptor. Biochem. J. 373:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okajima, K. 2004. Prevention of endothelial cell injury by activated protein C: the molecular mechanism(s) and therapeutic implications. Curr. Vasc. Pharmacol. 2:125-133. [DOI] [PubMed] [Google Scholar]
- 21.Poeze, M., G. Ramsay, H. Gerlach, F. Rubulotta, and M. Levy. 2004. An international sepsis survey: a study of doctors' knowledge and perception about sepsis. Critical Care (London) 8:R409-R413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rausa, F., Y. Tan, and R. Costa. 2003. Association between hepatocyte nuclear factor 6 (HNF-6) and FoxA2 DNA binding domains stimulates FoxA2 transcriptional activity but inhibits HNF-6 DNA binding. Mol. Cell. Biol. 23:437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riewald, M., R. Petrovan, A. Donner, B. Mueller, and W. Ruf. 2002. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296:1880-1882. [DOI] [PubMed] [Google Scholar]
- 24.Schmide-Supprian, M., C. Murphy, B. White, M. Lawler, A. Kapurniotu, W. Voelter, O. Smith, and J. Bernhagen. 2000. Activated protein C inhibits tumor necrosis factor and macrophage migration inhibitory factor production in monocytes. Eur. Cytokine Netw. 11:407-413. [PubMed] [Google Scholar]
- 25.Simpson, K. J., N. C. Henderson, C. L. Bone-Larson, N. W. Lukacs, C. M. Hogaboam, and S. L. Kunkel. 2003. Chemokines in the pathogenesis of liver disease: so many players with poorly defined roles. Clin. Sci. 104:47-63. [DOI] [PubMed] [Google Scholar]
- 26.Spek, C. A., J. S. Greengard, J. H. Griffin, R. M. Bertina, and P. H. Reitsma. 1995. Two mutations in the promoter region of the human protein C gene both cause type I protein C deficiency by disruption of two HNF-3 binding sites. J. Biol. Chem. 270:24216-24221. [DOI] [PubMed] [Google Scholar]
- 27.Spek, C. A., V. J. Lannoy, F. P. Lemaigre, G. G. Rousseau, R. M. Bertina, and P. H. Reitsma. 1998. Type I protein C deficiency caused by disruption of a hepatocyte nuclear factor (HNF)-6/HNF-1 binding site in the human protein C gene promoter. J. Biol. Chem. 273:10168-10173. [DOI] [PubMed] [Google Scholar]
- 28.Sturn, D. H., N. C. Kaneider, C. Feistritzer, A. Djanani, K. Fukudome, and C. J. Wiedermann. 2003. Expression and function of the endothelial protein C receptor in human neutrophils. Blood 102:1499-1505. [DOI] [PubMed] [Google Scholar]
- 29.Tsay, W., Y. M. Lee, S. C. Lee, M. C. Shen, and P. J. Chen. 1996. Characterization of human protein C gene promoter: insights from natural human mutants. DNA Cell Biol. 15:907-919. [DOI] [PubMed] [Google Scholar]
- 30.Tsay, W., Y. M. Lee, S. C. Lee, M. C. Shen, and P. J. Chen. 1997. Synergistic transactivation of HNF-1α, HNF-3, and NF-I contributes to the activation of the liver-specific protein C gene. DNA Cell Biol. 16:569-577. [DOI] [PubMed] [Google Scholar]
- 31.Uchiba, M., K. Okajima, Y. Oike, Y. Ito, K. Fukudome, H. Isobe, and T. Suda. 2004. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ. Res. 95:34-41. [DOI] [PubMed] [Google Scholar]