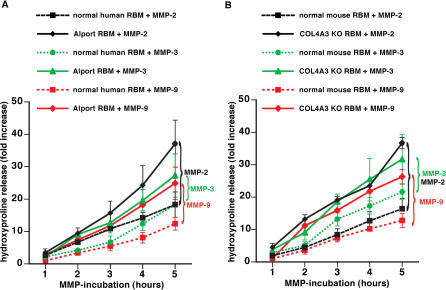
Figure 3. Increased Susceptibility to Proteolytic Degradation by MMPs of RBM from Patients with XAS and α3(IV) −/− Mice .
(A) Degradation of human RBM by MMP-2, MMP-3, and MMP-9. RBM was isolated from human control kidneys and from patients with XAS. RBM was incubated with MMPs, and proteolysis was assessed by estimation of hydroxyproline release. The graph displays the relative increase of hydroxyproline over time. Experiments using normal RBM are represented by dotted lines; studies with RBM from patients with XAS are represented by a full line. Hydroxyproline release after MMP-2 incubation is displayed by black lines, MMP-3 incubation by green lines, and MMP-9 incubation by red lines. Proteolysis of Alport BM was significantly enhanced compared with normal human RBM by each of these MMPS.
(B) Degradation of mouse RBM by MMP-2, MMP-3, and MMP-9. RBM was collected from normal C57BL/6 mice ( n = 6) and from α 3(IV) −/− mice ( n = 6) and subjected to active MMPs. Experiments using normal RBM are represented by dotted lines; studies with RBM from α3(IV) −/− mice are represented by a full line. Hydroxyproline release after MMP-2 incubation is displayed by black lines, MMP-3 incubation by green lines, and MMP-9 incubation by red lines. Similarly to human Alport RBM, the RBM from α3(IV) −/− mice displayed an increased susceptibility to proteolysis by MMPs.