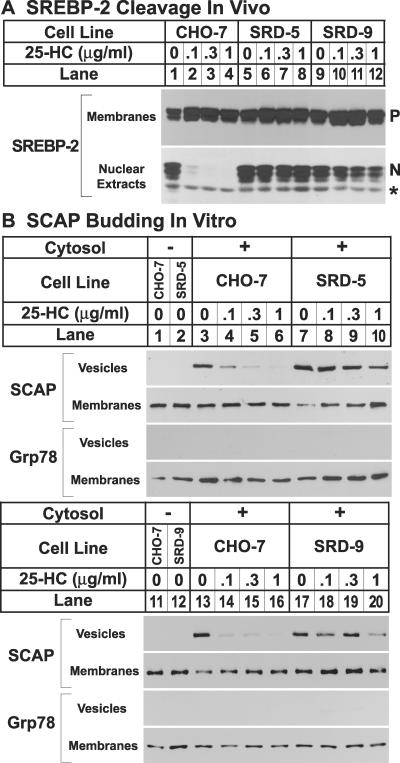
Fig 2.
(A) SREBP-2 cleavage in wild-type CHO-7 cells and mutant SRD-5 and SRD-9 cells. On day 0, cells were set up at the following densities: CHO-7, 5 × 105 cells per 100-mm dish; and SRD-5 and SRD-9, 8 × 105 cells per 100-mm dish. On day 2, cells were washed once with PBS and refed with medium C in the absence or presence of indicated concentrations of 25HC. Cells that received 25HC also received 10 μg/ml cholesterol. On day 3, the cells were harvested, and membranes and nuclear extracts were prepared as described (10). Aliquots of membranes (25 μg of protein) and nuclear extracts (50 μg of protein) were subjected to SDS/PAGE and immunoblot analysis with 5 μg/ml IgG-7D4 (SREBP-2). N and P denote the nuclear and precursor forms of SREBP-2, respectively. The asterisk (*) denotes a crossreacting protein of unknown identity. (B) Constitutive exit of SCAP from the ER in mutant SRD-5 and SRD-9 cells, as determined by in vitro vesicle-formation assay. On day 0, cells were set up at the following densities: CHO-7, 5 × 105 cells per 100-mm dish; SRD-5, 12 × 105 cells per 100-mm dish; and SRD-9, 8 × 105 cells per 100-mm dish. On day 3, the cells were switched to medium C containing 1% hydroxypropyl-β-cyclodextrin and incubated for 1 h at 37°C. Cells then were washed twice with PBS and switched to medium B in the absence or presence of the indicated concentrations of 25HC. Cells that received 25HC also received 10 μg/ml cholesterol. After incubation for 5 h at 37°C, cells were harvested for membrane preparation. Membranes were incubated in vitro under conditions that permit vesicle budding as described (3). Aliquots of the membranes (80 μg of protein) were incubated in the absence (lanes 1 and 2) or presence (lanes 3–10) of rat liver cytosol for 15 min at 28°C. Vesicle and membrane fractions were separated by centrifugation, and aliquots containing 100% of the vesicle protein and 20% of the membrane protein were subjected to SDS/PAGE and immunoblot analysis with IgG-9D5 (SCAP) and anti-KDEL antibody (grp78).