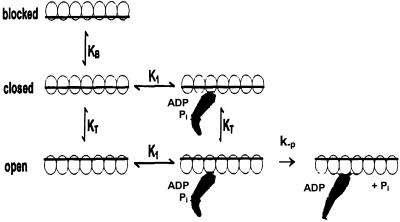
Fig 6.
Three-state steric blocking model applied to the regulation of phosphate dissociation. KB is the equilibrium constant between the blocked and closed states, KT the equilibrium constant between the blocked and open states, K1 the association constant of M-ADP-Pi binding to the open and closed states, and k−P the rate constant of dissociation of phosphate from AM-ADP-Pi. The dependence of kobs on k−P, K1, KT, KB, and [A] is: kobs = k−P/((1 + 1/KT) + (1/K1[A])(1 + (1/KT)(1 + 1/KB))) ≈ k−PKT/(1 + KT) for KB ≫1 and [A] ≫ K1.