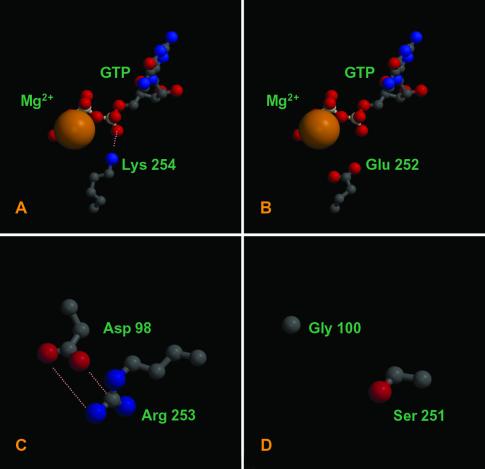
Fig 4.
Several notable changes are present at the P. dejongeii tubulin intradimer interface. Lysine residue 254, which neutralizes the N-site GTP phosphate groups (A) is highly conserved in eukaryotic β tubulin but is substituted for glutamic acid in BtubB (B). A key salt bridge at the interface of eukaryotic tubulins (C) is absent in the P. dejongeii tubulin dimer (D). BtubA and BtubB are >35% identical to bovine α and β tubulin. Models ranging from 1.5 to 2.5 Å rms deviation for this degree of sequence relationship are routinely produced using these methods (15, 16); thus, our models can be considered highly reliable. Figures were generated with MOLSCRIPT (21) and RASTER 3D (22).