Abstract
Since 2002, New Zealand's incidence of campylobacteriosis has exceeded 300 cases per 100,000 people per annum. To evaluate genetic variation in human isolates, 183 Campylobacter isolates were collected from a single clinical laboratory in Christchurch: 77 during an 8-week period in spring, and the rest 3 months later over a second 8-week period in autumn. Isolates were identified to the species level and subtyped using Penner serotyping (Campylobacter jejuni only) and pulsed-field gel electrophoresis (PFGE) using both SmaI and KpnI. Approximately two-thirds of the isolates could be grouped into clusters of between 2 and 26 isolates with indistinguishable SmaI and KpnI patterns. Less than 10% of the isolates were of the same type between the two sampling periods. The epidemiological relevance of the PFGE clusters was supported by temporal clustering, some spatial clustering, and some statistically significant demographic similarities among cases in a cluster. Conversely, patient cases yielding isolates which did not cluster with isolates from other cases were more likely to report recent overseas travel and less likely to live within larger urban centers. To identify whether these clusters actually represent common-source outbreaks, however, would require the detailed, rapid, and reiterative epidemiological investigation of cases within a PFGE cluster. The combined and timely application of subtyping and epidemiological investigation would appear to be a promising strategy for understanding campylobacteriosis in New Zealand.
The identification and investigation of disease outbreaks—which can each be defined as two or more cases thought to be linked by a common exposure—has been invaluable for understanding and combating many diseases. Campylobacteriosis has emerged worldwide as a significant cause of gastric illness, and New Zealand has one of the highest rates of campylobacteriosis in the developed world, with 327.4 cases per 100,000 people notified in 2004 (1). While outbreaks of campylobacteriosis have been identified both in New Zealand and elsewhere (8, 12, 14, 36), they generally account for only a small proportion of cases and, as a consequence, campylobacteriosis has been described as predominantly a sporadic disease (23, 25) for which the investigation of outbreaks is of limited value (15, 23).
Most recognized cases of campylobacteriosis are caused by infection with Campylobacter jejuni, with a smaller proportion caused by Campylobacter coli (25). Species of Campylobacter can be carried by a range of animal species including farm animals, wild birds, and pets (6, 7, 9, 29) and spread via contaminated food, milk, water, and even flies (11). An increasing awareness has emerged of the importance of, first, identifying Campylobacter to the species level (17) and second, applying appropriate subtyping methodology (35). Penner serotyping of C. jejuni and C. coli has been used for many years (21), and a range of molecularly-based subtyping approaches have been developed, including pulsed-field gel electrophoresis (PFGE) (30), multilocus sequence typing (10), fla typing, and AFLP (26).
In this pilot study we sought first to evaluate the range of Penner serotypes and PFGE types present among notified human isolates from one defined geographical area in New Zealand and in two defined time periods. The null hypothesis was that all or most isolates would be different, limiting any potential application of subtyping to identify and delineate clusters of Campylobacter cases. Second, epidemiological data for notified cases subtyped in this study were examined in a preliminary evaluation of the potential significance or relevance of any clustering of isolates observed.
MATERIALS AND METHODS
Campylobacter isolates were obtained from 183 human fecal samples submitted to a clinical laboratory in Christchurch, New Zealand, which primarily processes fecal samples referred from community general practitioners. These isolates were temporally separated, with the first 77 collected over an 8-week period in spring 2002 (laboratory testing dates of 9 September to 13 November 2002, weeks 1 to 8), and the second set of 106 collected 15 weeks later in autumn 2003 (laboratory testing dates of 26 February to 17 April 2003, weeks 23 to 30). The isolates were obtained from fecal samples streaked onto charcoal cefoperazone deoxycholate agar that were incubated microaerobically at 37°C for 48 h. Colonies that were suggestive of Campylobacter were confirmed as gram-negative curved bacilli by Gram stain. Isolates were then restreaked on Columbia sheep blood agar, identified as either C. jejuni or C. coli using a multiplex PCR assay (37), and frozen at −80°C.
All isolates were analyzed by PFGE using the standardized PulseNet protocol (30), with the Salmonella Braenderup H9812 strain restricted with XbaI as a size standard (16). Gels were made with 1% (wt/vol) SeaKem Gold agarose and electrophoresed for 18 h using an initial switch time of 6.8 s and a final switch time of 38.4 s for SmaI and an initial switch time of 5.2 s and a final switch time of 42.3 s for KpnI. PFGE profiles were analyzed and compared using BioNumerics version 4.0 (Applied Maths, Ghent, Belgium). Isolates were submitted to the PulseNet Aotearoa New Zealand Campylobacter database, where SmaI and KpnI pattern designations were assigned. PFGE clusters were defined as isolates with indistinguishable SmaI and KpnI patterns. These PFGE clusters were designated with a single letter (A through X) for PFGE clusters within either of the sampling periods, while PFGE cluster designations AA through EE were assigned to isolates observed only once in both sampling periods. Heat-stable (HS) Penner serotypes were determined using a panel of 43 C. jejuni antisera produced in-house according to the method of Penner and Hennessy (28).
Isolates were matched to notified cases in the New Zealand EpiSurv notified diseases database using data provided by the clinical laboratory. Home addresses corresponding to the notified cases were mapped using ArcView version 8.2 (ESRI, Redlands, California). Christchurch City cases were defined as those whose patients had home addresses within a 15-km diameter of the city center. Odds ratios (OR) and 95% confidence intervals (CI) (3) were calculated for cases of the same PFGE type using, as the control group, cases of a different PFGE type from the same sampling period, unless otherwise specified.
RESULTS
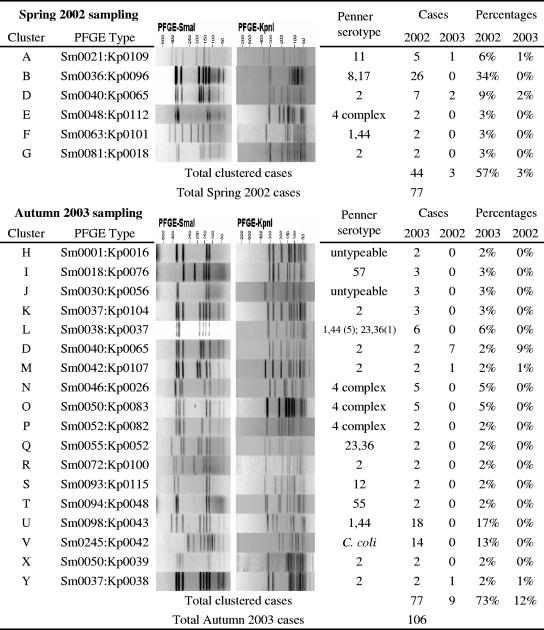
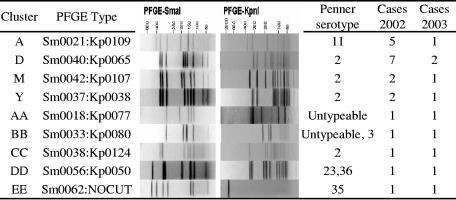
Multiplex PCR analysis identified 168 of the isolates as C. jejuni and 15 isolates as C. coli. All of the C. coli isolates were recovered in the second sampling period. SmaI PFGE patterns were generated for all 183 isolates, with 57 different patterns observed. Three of the isolates could not be restricted with KpnI, but of the remaining 180 isolates, 71 different KpnI patterns were generated. When combined, 77 different SmaI-KpnI combinations were observed. Forty-nine of the isolates produced SmaI-KpnI profiles which were observed only once in the study. The remaining isolates formed PFGE clusters of between 2 and 26 isolates (Fig. 1), with 57% of the isolates in weeks 1 to 8 and 73% of the isolates in weeks 23 to 30 forming PFGE clusters. Just nine PFGE subtypes representing 31 isolates were observed in both sampling periods (Fig. 2). Penner serotyping of the 168 C. jejuni isolates identified 17 different serotypes (Table 1). The four most common serotypes observed (2; 1,44; 8,17; and 4 complex) accounted for 71% of the isolates in this study. Except for serotypes 8,17 and 11, and those serotypes observed only once, all serotypes could be distinguished into multiple PFGE types, with relatively high diversity indices (Table 1). Among the PFGE groupings, except for two instances, all isolates within a cluster had the same Penner serotype.
FIG. 1.
PFGE clusters observed in the two sampling periods.
FIG. 2.
PFGE types observed in both sampling periods.
TABLE 1.
Variation in PFGE types observed among the C. jejuni Penner serotypes
| Serotype | Count | % | PFGE types | Diversity |
|---|---|---|---|---|
| 2 | 39 | 23 | 21 | 0.54 |
| 1,44 | 29 | 17 | 8 | 0.28 |
| 8,17 | 26 | 15 | 1 | 0.04 |
| 4c | 26 | 15 | 15 | 0.58 |
| 11 | 6 | 4 | 1 | 0.17 |
| 23,36 | 6 | 4 | 5 | 0.83 |
| 12 | 3 | 2 | 2 | 0.67 |
| 35 | 3 | 2 | 2 | 0.67 |
| 37 | 3 | 2 | 3 | 1.00 |
| 57 | 3 | 2 | 3 | 1.00 |
| 3 | 2 | 1 | 2 | 1.00 |
| 9 | 2 | 1 | 2 | 1.00 |
| 21 | 2 | 1 | 2 | 1.00 |
| 5 | 1 | 1 | 1 | 1.00 |
| 15 | 1 | 1 | 1 | 1.00 |
| 45 | 1 | 1 | 1 | 1.00 |
| 55 | 1 | 1 | 1 | 1.00 |
| Untypeable | 14 | 8 | 10 | 0.71 |
| Total | 168 | 81 |
It was possible to match 165 of the 183 isolates (90%) to notified cases in the New Zealand EpiSurv notified diseases database. Using the case notification date for comparison, these cases represented 29% of the total notified cases in this region for each sampling period. The ages of the patients in the cases ranged from 8 months to 80 years. Females comprised 51% of cases, and the ethnicity of patients in the cases was 92% European, 3% Maori, and 5% other.
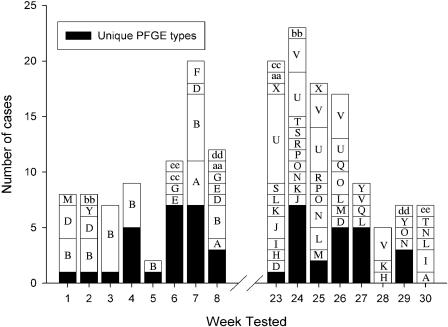
Due to incomplete case histories, identifying common links between cases was difficult, and a common source between cases was not identified for any of the PFGE clusters. However, the epidemiological relevance of the PFGE clusters was supported by a number of similarities observed between cases in the three largest PFGE clusters, cases in some of the smaller PFGE clusters, and, also, the unique isolates. Temporal clustering was observed for almost all of the isolates within a PFGE cluster (Fig. 3). For example, all 18 cases in the U cluster occurred within a 4-week period at the start of the autumn sampling period, and both cases in the F cluster were isolated in the same week (Fig. 3).
FIG. 3.
Temporal clustering of Campylobacter isolates.
B cluster.
One-third of the isolates (26 of 77) in the spring 2002 sampling period were of the same PFGE type (Sm0036:Kp0096) and all were Penner serotype HS:8,17 (Table 1). This PFGE type was not seen among any of the autumn 2003 isolates. Onset dates were recorded for 18 of these cases and ranged between 18 September 2002 and 5 November 2002. Two of the cases represented married patients, with an onset date one day apart, suggesting both are primary cases. Patients from eight of the cases did not respond to questionnaires, and the patient from one case was hospitalized. Home addresses were available for patients from 24 of the B cluster cases, who were more likely to reside within the Christchurch City region (OR = 6.2, 95% CI = 2.0 to 18.9). Ten of the cases were patients who reported contact with a dog and/or a cat (OR = 3.4, CI = 1.1 to 10.3).
U cluster.
Eighteen cases in autumn 2003 had the PFGE profile Sm0098:Kp0043. All were serotype 1,44. Onset dates of the cases were between 15 February 2003 and 5 March 2003 (5 were of unknown onset date). Patients from three of the cases reported friends or family with symptoms, none of which were included in this study. The occupations of patients in 16 of the cases were available, and 4 were students at the same tertiary educational institute. None of the other patients whose cases were in the study listed their occupation as being at thesame institute. Using “Campylobacter of subtype U” as the case definition and “student at this institute” as the occupation produces an odds ratio of 55 (95% CI = 2.8 to 1,075). Patients from five of the other cases with subtype U had occupations at similar tertiary institutes and hospitals. The ages of the patients in 13 of the cases in the U cluster were between 19 and 36 years, suggesting an age-related link between cases (OR = 11.7, CI = 3.4 to 40.4).
V cluster.
Fourteen cases in autumn 2003 had the PFGE profile Sm00245:Kp0042 and represented 14 of the 15 C. coli isolates identified (none were identified from the 2002 isolates). Two of these cases were not notified, and the patients from three did not respond to questionnaires. Isolates in this cluster had onset dates between 20 February 2003 and 11 March 2003. Eight of the 12 cases providing age data were from patients more than 37 years old (OR 2.4, CI 0.7 to 8.9). Patients from four of the nine cases reported recreational water contact, with three different swimming pools and one stream (OR 5.2, CI 1.1 to 23.6).
Smaller clusters.
For many of the two- to three-case clusters, data were only available for one of the cases, making any interpretation impossible. For several of the small clusters, patients from the cases had similar ages. For example, cases in Q and I clusters (two each) were for patients both over 55 (OR = 16.9, CI = 0.8 to 364), R cluster case patients were aged 19 and 22 (OR = 80, CI = 3.4 to 1,889), and the patients from Y cluster cases were 9 and 12 years old (OR = 99, CI = 4.1 to 2,392). Patients from four of the six isolates in the D cluster lived in rural towns (OR = 1.9, CI = 0.3 to 11.0) as did those for four of the five cases in cluster A (OR = 3.9, CI = 0.4 to 36.6). Within the Christchurch City area some suggestive, although not conclusive, clustering of PFGE types was observed, with, for example, the patients from the two cases in cluster F living within 2.5 km of each other.
Unique isolates.
Isolates which did not cluster with any others were more common from cases of patients not residing in the Christchurch City area (OR = 1.7, CI = 0.9 to 3.4). A response was recorded for patients from 150 of the cases regarding their recent travel, of which all 4 cases with travel beyond Australia and New Zealand had unique types of Campylobacter isolates (OR = 19, CI = 1.0 to 368).
DISCUSSION
Subtyping analysis.
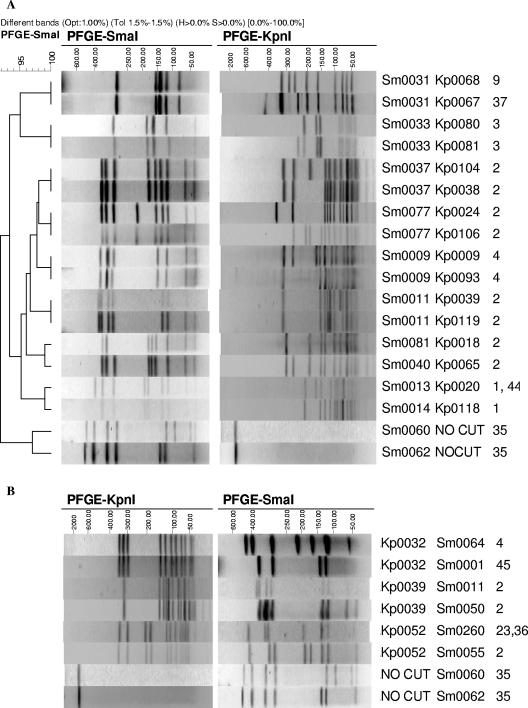
The most important finding of this study was that up to two-thirds of the isolates examined can be grouped into clusters of two or more isolates with indistinguishable PFGE profiles. Less than 30% of the notified cases in the time periods examined were actually part of this study, suggesting that examination of isolates from all cases would produce both additional and larger clusters of PFGE types. The definition of a subtype cluster used in this study was indistinguishable PFGE patterns with both SmaI and KpnI restriction enzymes. The combination of Penner serotyping and PFGE (usually with SmaI) has been used and recommended by a number of researchers (9, 27, 31). While Penner serotyping did break down some SmaI types in this study, KpnI digestion provided better discrimination both to identify differences and confirm similarities (Fig. 4A). Penner serotyping provided no additional level of discrimination beyond that which SmaI and KpnI digestion provided.
FIG. 4.
PFGE profiles of selected isolates. (A) Illustration of the value of KpnI to distinguish SmaI patterns and to confirm relatedness among similar isolates. (B) Shown, conversely, are isolates indistinguishable using KpnI that can be distinguished using SmaI digestion.
Digestion with KpnI was almost as discriminatory as SmaI and KpnI combined, suggesting that digestion with KpnI alone could be an effective approach, a conclusion also supported by Michaud et al. (24). In addition, the cost of the KpnI enzyme is less than 30% of the cost of SmaI, reducing the overall consumables cost of PFGE with KpnI to almost half that of PFGE with both SmaI and KpnI. However, even among the limited number of isolates in this study, isolates with indistinguishable or similar KpnI patterns can be further subgrouped when analyzed with SmaI (Fig. 4B). Internationally, most Campylobacter PFGE data have been generated using SmaI as the primary enzyme (PulseNet, CampyNet), perhaps partly because KpnI is a more difficult enzyme with which to achieve reproducible results. SmaI digestion, while less discriminatory, is sufficient in many cases to demonstrate that isolates are different. We believe that to demonstrate similarity, or that isolates are indistinguishable, digestion with two enzymes is essential, a finding also supported by other researchers (20, 27, 31). With sufficient international data, a reconsideration of using KpnI as the primary enzyme could be made in the future and useful comparisons with existing data still made.
Epidemiological analysis.
In this study, 90% of isolates could be linked retrospectively to notified campylobacteriosis cases, which is a rate 10% higher than that reported previously in the Auckland area of New Zealand (33). The epidemiological information associated with these notified cases was obtained retrospectively from data submitted to the New Zealand EpiSurv databases. These data are entered by local public health units based primarily on general practitioner notifications and postal questionnaires filled out by the patients in the cases themselves. No effort was made in this study to improve the quality of this initial data collection, nor to follow up potential clusters of isolates. Consequently the epidemiological data associated with these notified cases were very incomplete with, for example, only 75% of the notified cases in this study having an onset date recorded. Identifying actual common sources of Campylobacter between cases in a cluster was not possible, and this study was not designed for that objective. Examination of the cases did, however, identify a number of commonalities between cases in a PFGE cluster, including temporal and spatial linkages. The use of geographic information system mapping could be especially useful, particularly if, in addition to home addresses, the place of work or school, main shopping areas, and restaurants frequented were also mapped. A number of demographic features were also shared by some cases in a PFGE cluster, including ages and, interestingly for the U cluster, occupations.
The value of subtyping is aptly illustrated when the correlation observed between subtype U cases and the occupation category (at a specific university) is considered without subtyping. In a situation where the case definition was simply campylobacteriosis, there would be patients from four cases that attend this university and 85 that do not. If a case-control study were to be conducted on the 89 cases, then we would require 89 matching controls. Based on a population in the study area of 450,000 and a university population of 10,000, then at least two of the controls would, by chance, be university students. An odds ratio in this case would be just 2.0 (95% CI = 0.4 to 11.5), rather than 51 (95% CI = 2.6 to 1024), which the more specific case definition of subtype U provides. If actual common sources are identified, subtyping will be essential to support investigative suspicions.
No C. coli isolates were identified in the first sampling period, but 15 were recovered in the second sampling period, 14 of which were of the same PFGE type. The suggested association of this cluster with recreational water contact is interesting, since C. coli is often associated with water (7, 13, 19). Pigs and sheep have also been identified to have relatively higher prevalence of C. coli (7). Recreational water contact has been previously identified as a source of campylobacteriosis both in New Zealand (4, 34) and overseas (32). The patients from the four cases involved had recreational contact with four different rivers, lakes, or swimming pools. Therefore, if recreational water contact was a source, it would suggest that this C. coli type is very common in New Zealand. We have insufficient data on C. coli PFGE types in New Zealand to make that assessment. Alternatively, recreational water contact may in fact be a surrogate of a shared lifestyle, which is reflected in some other unidentified common source for the cases in this cluster.
Case reports for many of the cases noted a number of food risk factors and suspected sources including particular foods eaten and restaurants frequented. Except in a very broad sense (e.g., chicken eaten) there were no direct matches in risk exposures for cases in a PFGE cluster. The specific risk factors noted do, however, provide numerous clues or starting points for secondary interviews of cases where the use of specific questions could more effectively identify or eliminate suspected sources. This type of approach has proven useful in interviews to identify sexual partners (5), is part of the cognitive interview technique used in criminal investigations (22), and is being applied to food-borne disease investigations (18).
PFGE cluster N (Sm0046:Kp0026) is indistinguishable from a familial common-source outbreak linked to precooked sausages distributed by a particular butcher in Christchurch (14). These outbreak isolates were recovered 1 month after the final N cluster isolates, i.e., outside the sampling period of this study. It is possible that this described outbreak was actually larger and occurred over a longer time than reported by Graham et al. (14).
Michaud et al. (23) examined by KpnI PFGE 183 isolates of the 201 reported cases of campylobacteriosis in Quebec, Canada, over a 15-month period (rate, 63.1 cases per 100,000 people). They found 55% formed KpnI PFGE clusters of between 2 and 11 isolates (Dice similarity of 0.9) but found few epidemiological links and concluded that “molecular typing identifies relatively few additional cases representing potential common-source clusters.” Hedberg et al. (15) reported that, of the 941 cases of campylobacteriosis reported among Minnesota residents in 1994, subtyping of 673 of these by PFGE identified 248 distinct PFGE patterns, 74% of which were represented by only one or two isolates. Most (87%) isolates could not be linked by time, geographic location, or PFGE type. They concluded that the large diversity of PFGE patterns limits the usefulness of PFGE for outbreak detection. In comparison, we found an apparently higher number of isolates belonging to subclusters. The rate of campylobacteriosis in the New Zealand study area is at least five times higher than in either Quebec or Minnesota. Whether this is due to a greater number of common-source outbreaks (and hence the higher proportion of isolates in clusters that we found) or whether other factors resulted in a higher proportion of cases being captured by the laboratory and surveillance system is an unresolved question. Whatever the explanation, New Zealand's high number of cases may, with the application of subtyping, make the identification of common-source outbreaks more practical. In this study, however, we were no more successful in identifying common sources than the researchers in the two studies cited above. We would suggest that this is not because those common sources do not exist, but because of limitations in the quality, quantity, and timeliness of the epidemiological data that were collected. For any disease, finding epidemiological links between cases is often a difficult and resource-intensive exercise. This is particularly so for Campylobacter cases which, although typically having an incubation period of 2 to 5 days, may have an incubation period extending up to 11 days (2, 25), with additional time delays until medical examination is sought, samples are analyzed, notifications occur, and investigations begin. The multitude of potential exposures and sources for Campylobacter infection complicates things further. Together with imperfect recall by the patients in cases, the possibility that some cases may be secondary to those with the primary exposure and the possibility that a case's patient may not have consumed the primary contaminated product but may have handled it or been exposed through cross-contamination will make identifying common sources difficult. Indeed, some clusters of types may not even have a common source if they represent a stable, endemic type, although this pilot study produced little evidence of this in New Zealand. If, as we suggest, PFGE clusters represent potential common-source outbreaks, finding common epidemiological linkages will be a difficult, but not impossible, task.
This study demonstrates that for New Zealand Campylobacter isolates, PFGE analysis is able to cluster isolates that potentially represent common-source outbreaks. Despite limited and incomplete epidemiological information for each case, there was temporal, spatial, and demographic support for this hypothesis. We now propose genotyping all human isolates and targeted isolates from potential sources in defined temporal and spatial areas, in conjunction with prompt and reiterative investigation of clusters of cases. This approach may help identify and quantify the actual causes and sources of the campylobacteriosis in New Zealand and therefore provide support for appropriate interventions.
Acknowledgments
We acknowledge funding from the New Zealand Food Safety Authority for the initial collection and partial analysis of isolates and from the ESR Internal Reinvestment Fund for completion of this study.
We thank Sue Walker for Penner serotyping of isolates and Andrew Hudson, Meg Devane, Rosemary Whyte, Andrew Ball, and Teck Lok Wong from ESR for useful discussions and review of the manuscript. We acknowledge also the helpful discussion held with Chris Carlson, South Dakota Department of Health, and Stephanie Wedel, Minnesota Department of Health.
REFERENCES
- 1.Anonymous. 2005. Notifiable and other diseases in New Zealand. Annual Report 2004. [Online.] ESR Limited, Wellington, New Zealand. http://surv.esr.cri.nz/surveillance/annual_surveillance.php.
- 2.Anonymous. 2004. Second report on Campylobacter. [Online.] Advisory Committee on the Microbiological Safety of Food, London, United Kingdom. http://www.food.gov.uk/multimedia/pdfs/acmsfcampyloreport.pdf.
- 3.Bhopal, R. 2002. Concepts of epidemiology: an integrated introduction to the ideas, theories, principles and methods of epidemiology. Oxford University Press, New York, N.Y.
- 4.Bohmer, P. 1997. Outbreak of campylobacteriosis at a school camp linked to water supply. N. Z. Public Health Rep. 4:58-59. [Google Scholar]
- 5.Brewer, D. D., J. J. Potterat, S. Q. Muth, P. Z. Malone, P. Montoya, D. L. Green, H. L. Rogers, and P. A. Cox. 2005. Randomized trial of supplementary interviewing techniques to enhance recall of sexual partners in contact interviews. Sex. Transm. Dis. 32:189-193. [DOI] [PubMed] [Google Scholar]
- 6.Broman, T., H. Palmgren, S. Bergstrom, M. Sellin, J. Waldenstrom, M. L. Danielsson-Tham, and B. Olsen. 2002. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J. Clin. Microbiol. 40:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, P. E., O. F. Christensen, H. E. Clough, P. J. Diggle, C. A. Hart, S. Hazel, R. Kemp, A. J. Leatherbarrow, A. Moore, J. Sutherst, J. Turner, N. J. Williams, E. J. Wright, and N. P. French. 2004. Frequency and spatial distribution of environmental Campylobacter spp. Appl. Environ. Microbiol. 70:6501-6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, C. G., L. Price, R. Ahmed, D. L. Woodward, P. L. Melito, F. G. Rodgers, F. Jamieson, B. Ciebin, A. Li, and A. Ellis. 2003. Characterization of waterborne outbreak-associated Campylobacter jejuni, Walkerton, Ontario. Emerg. Infect. Dis. 9:1232-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devane, M. L., C. Nicol, A. Ball, J. D. Klena, P. Scholes, J. A. Hudson, M. G. Baker, B. J. Gilpin, N. Garrett, and M. G. Savill. 2005. The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. J. Appl. Microbiol. 98:980-990. [DOI] [PubMed] [Google Scholar]
- 10.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. L. Willems, R. Urwin, and M. C. J. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekdahl, K., B. Normann, and Y. Andersson. 2005. Could flies explain the elusive epidemiology of campylobacteriosis? BMC Infect. Dis. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald, C., L. O. Helsel, M. A. Nicholson, S. J. Olsen, D. L. Swerdlow, R. Flahart, J. Sexton, and P. I. Fields. 2001. Evaluation of methods for subtyping Campylobacter jejuni during an outbreak involving a food handler. J. Clin. Microbiol. 39:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie, I. A., S. J. O'Brien, J. A. Frost, G. K. Adak, P. Horby, A. V. Swan, M. J. Painter, and K. R. Neal. 2002. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg. Infect. Dis. 8:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham, C. F., R. Whyte, B. J. Gilpin, A. J. Cornelius, J. A. Hudson, D. Morrison, H. Graham, and C. Nicol. 2005. Outbreak of campylobacteriosis following pre-cooked sausage consumption. Aust. N. Z. J. Public Health 29:507-510. [DOI] [PubMed] [Google Scholar]
- 15.Hedberg, C. W., K. E. Smith, J. M. Besser, D. J. Boxrud, T. W. Hennessy, J. B. Bender, F. A. Anderson, and M. T. Osterholm. 2001. Limitations of pulsed-field gel electrophoresis for the routine surveillance of Campylobacter infections. J. Infect. Dis. 184:242-244. [DOI] [PubMed] [Google Scholar]
- 16.Hunter, S. B., P. Vauterin, M. A. Lambert-Fair, M. S. Van Duyne, K. Kubota, L. Graves, D. Wrigley, T. Barrett, and E. Ribot. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klena, J. D., C. T. Parker, K. Knibb, J. C. Ibbitt, P. M. L. Devane, S. T. Horn, W. G. Miller, and M. E. Konkel. 2004. Differentiation of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis by a multiplex PCR developed from the nucleotide sequence of the lipid A gene lpxA. J. Clin. Microbiol. 42:5549-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laine, E. S., J. M. Scheftel, D. J. Boxrud, K. J. Vought, R. N. Danila, K. M. Elfering, and K. E. Smith. 2005. Outbreak of Escherichia coli O157:H7 infections associated with nonintact blade-tenderized frozen steaks sold by door-to-door vendors. J. Food Prot. 68:1198-1202. [DOI] [PubMed] [Google Scholar]
- 19.Leatherbarrow, A. J. H., C. A. Hart, R. Kemp, N. J. Williams, A. Ridley, M. Sharma, P. J. Diggle, E. J. Wright, J. Sutherst, and N. P. French. 2004. Genotypic and antibiotic susceptibility characteristics of a Campylobacter coli population isolated from dairy farmland in the United Kingdom. Appl. Environ. Microbiol. 70:822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindmark, H., B. Harbom, L. Thebo, L. Andersson, G. Hedin, B. Osterman, T.Lindberg, Y. Andersson, A. Westöö, and E. Olsson Engvall. 2004. Genetic characterization and antibiotic resistance of Campylobacter jejuni isolated from meats, water, and humans in Sweden. J. Clin. Microbiol. 42:700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKay, D., J. Fletcher, P. Cooper, and F. M. Thomson-Carter. 2001. Comparison of two methods for serotyping Campylobacter spp. J. Clin. Microbiol. 39:1917-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memon, A., L. Wark, A. Holley, R. Bull, and G. Koehnken. 1997. Eyewitness performance in cognitive and structured interviews. Memory 5:639-656. [DOI] [PubMed] [Google Scholar]
- 23.Michaud, S., S. Menard, and R. D. Arbeit. 2005. Role of real-time molecular typing in the surveillance of Campylobacter enteritis and comparison of pulsed-field gel electrophoresis profiles from chicken and human isolates. J. Clin. Microbiol. 43:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaud, S., S. Menard, C. Gaudreau, and R. D. Arbeit. 2001. Comparison of SmaI-defined genotypes of Campylobacter jejuni examined by KpnI: a population-based study. J. Med. Microbiol. 50:1075-1081. [DOI] [PubMed] [Google Scholar]
- 25.Moore, J. E., D. Corcoran, J. S. Dooley, S. Fanning, B. Lucey, M. Matsuda, D. A. McDowell, F. Megraud, B. C. Millar, R. O'Mahony, L. O'Riordan, M. O'Rourke, J. R. Rao, P. J. Rooney, A. Sails, and P. Whyte. 2005. Campylobacter. Vet. Res. 36:351-382. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen, E. M., J. Engberg, V. Fussing, L. Petersen, C. H. Brogren, and S. L. On. 2000. Evaluation of phenotypic and genotypic methods for subtyping Campylobacter jejuni isolates from humans, poultry, and cattle. J. Clin. Microbiol. 38:3800-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.On, S. L. W., E. M. Nielsen, J. Engberg, and M. Madsen. 1998. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol. Infect. 120:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penner, J. L., and J. N. Hennessy. 1980. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J. Clin. Microbiol. 12:732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen, L., E. M. Nielsen, J. Engberg, S. L. On, and H. H. Dietz. 2001. Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl. Environ. Microbiol. 67:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito, S., J. Yatsuyanagi, S. Harata, Y. Ito, K. Shinagawa, N. Suzuki, K. Amano, and K. Enomoto. 2005. Campylobacter jejuni isolated from retail poultry meat, bovine feces and bile, and human diarrheal samples in Japan: comparison of serotypes and genotypes. FEMS Immunol. Med. Microbiol. 45:311-319. [DOI] [PubMed] [Google Scholar]
- 32.Schonberg-Norio, D., J. Takkinen, M. L. Hanninen, M. L. Katila, S. S. Kaukoranta, L. Mattila, and H. Rautelin. 2004. Swimming and Campylobacter infections. Emerg. Infect. Dis. 10:1474-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons, G., R. Whittaker, K. Boyle, A. J. Morris, A. Upton, and L. Calder. 2002. Could laboratory-based notification improve the control of foodborne illness in New Zealand? N. Z. Med. J. 115:237-240. [PubMed] [Google Scholar]
- 34.Stehr-Green, J. K., C. Nicholls, S. McEwan, A. Payne, and P. Mitchell. 1991. Waterborne outbreak of Campylobacter jejuni in Christchurch: the importance of a combined epidemiologic and microbiologic investigation. N. Z. Med. J. 104:356-358. [PubMed] [Google Scholar]
- 35.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whyte, R. J., J. A. Hudson, D. Morrison, and P. Burt. 2001. Outbreak of campylobacteriosis from chicken liver pāté. J. N. Z. Inst. Environ. Health 24:9-10. [Google Scholar]
- 37.Wong, T., M. Devane, J. A. Hudson, P. Scholes, M. Savill, and J. Klena. 2004. Validation of a PCR method for Campylobacter detection on poultry packs. Br. Food J. 106:642-650. [Google Scholar]