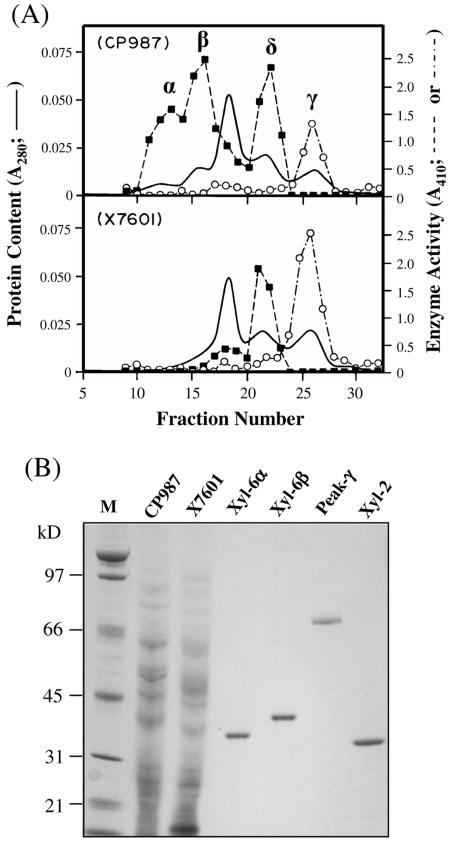
FIG. 3.
Purification of XYL-6p. (A) Cation-exchange chromatograms of secreted proteins. Secreted protein samples from 200 ml of fungal cultures were processed as previously described (35). The protein samples (∼2 mg each) were subjected to cation-exchange chromatography using a HiTrap SP column (Pharmacia Biotech) at pH 5.0. The column-bound proteins were eluted with a linear salt gradient from 0 to 1.0 m sodium chloride (fractions 1 to 120). Peak α was eluted by ∼0.045 M NaCl; peak β was eluted by ∼0.065 M NaCl; peak δ was eluted by ∼0.09 M NaCl, and peak λ was eluted by ∼0.11 M NaCl. A fifth peak eluted by ∼0.6 M NaCl and present in both CP987 and X7601 is not shown in this figure. This fifth peak contained an endo-β-1,4-xylanase, XYL-3, that has been described previously (37). Each fraction was assayed for endo-β-1,4-xylanase (▪) and endo-β-1,4-glucanase (○) activities using oat spelt xylan (Sigma catalog no. X-0627) dissolved in 100 mM 2-(N-morpholino)-ethane sulfonic acid buffer (pH 6.0) and carboxymethylcellulose (Sigma catalog no. C-0806) dissolved in 50 mM sodium acetate buffer (pH 5.0), respectively, as the substrates (see Materials and Methods) (35). (B) Gel analysis of the purified enzymes. Samples CP987 (2 μg) and X7601 (2 μg) are total extracellular proteins from the culture filtrate of M. grisea strains CP987 and X7601, respectively. XYL-6α (100 ng) and XYL-6β (100 ng) were purified, respectively, from peaks α and β shown in panel A by hydrophobic interaction chromatography (see Materials and Methods). Peak γ (40 ng) is the endo-β-1,4-glucanase-containing peak shown in panel A. XYL-2 (100 ng) is a 33-kDa xylanase purified previously (35). The protein samples were separated by SDS-PAGE in a 4 to 12% NuPAGE Bis-Tris precast gel (Invitrogen) as previously described (35, 37) and stained with a Colloidal Blue Staining kit (Invitrogen). Lane M, molecular mass ladder.