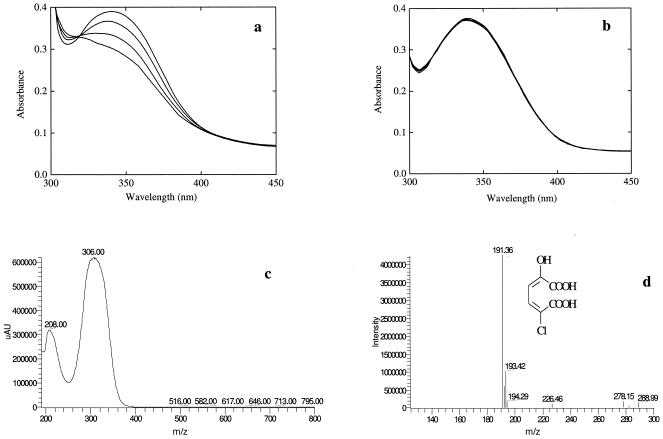
FIG. 2.
Conversion of 2-amino-5-chloromuconic acid (2A5CM) into 2-hydroxy-5-chloromuconic acid (2H5CM), catalyzed by recombinant E. coli/pETcnbH that expressed 2-amino-5-chloromuconic acid deaminase (CnbH) (a) and by E. coli/pET28a as a control (b), and photospectrometry of 2-hydroxy-5-chloromuconic acid (c) and mass spectrometry of 2-hydroxy-5-chloromuconic acid (d). The photospectrum in a and b was recorded at 0 to 90 min after addition of cellular lysate. The specific activities for 2-aminomuconic acid were calculated to be 7 nmol min−1 (mg of protein)−1 for recombinant E. coli expressing CnbH and 120 nmol min−1 (mg of protein)−1 for Comamonas sp. strain CNB-1.