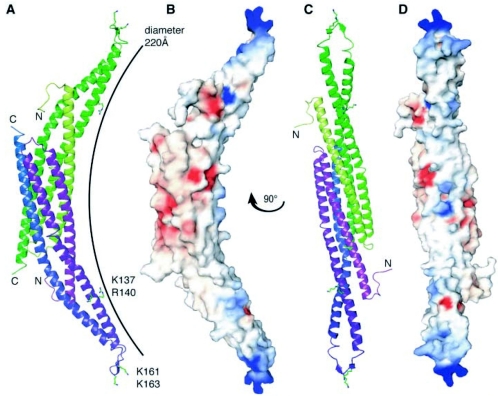
FIG. 13.
Three-dimensional crystal structure of the Drosophila amphiphysin BAR domain. A, Two amphiphysin monomers (depicted in purple and green) associate in antiparallel orientation to form a banana-shaped homodimer represented here in ribbon format. The residues that form the basic patches on the concave membrane-binding surface of the dimer are highlighted. The Protein Data Bank identifier is 1URU. Note that the Drosophila amphiphysin BAR domain lacks an NTID, but sequence alignment with mammalian Bin1 suggests the NTID would be positioned within the unstructured loop between helices 2 and 3 as seen in the Drosophila BAR domain. This loop is positively charged and two conserved lysine residues have been shown to be important for membrane association of both Drosophila amphiphysin and rat amphiphysins 1 and 2 (237). The presence of the NTID would contribute extra positive charges to the unstructured loop and may further strengthen membrane association. B, The homodimer represented in electrostatic surface format and viewed with the same orientation as in A. Electrostatic potential is indicated: −10 kTe−1 in red; +10 kTe−1 in blue). C, The homodimer represented in ribbon format but viewed at an angle perpendicular to that in panel A to highlight details of the concave face. D, The homodimer represented in electrostatic surface format and viewed with the same orientation as in panel C. (Reprinted from reference 237 with permission of the publisher. Copyright 2004 American Association for the Advancement of Science.)