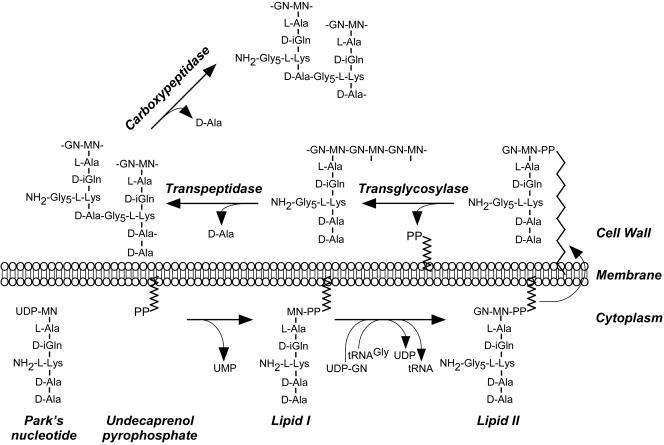
FIG. 1.
Peptidoglycan synthesis in S. aureus. Park's nucleotide, a soluble nucleotide precursor, originates in the bacterial cytoplasm by successive addition of l-stereoisomer amino acids (l-Ala and l-Lys) as well as d-stereoisomer amino acids (d-isoglutamine [d-iGln] and d-Ala) to UDP-N-acetylmuramic acid (UDP-NM). Precursor transfer to undecaprenol pyrophosphate, a bacterial membrane carrier, generates lipid I and removes UMP nucleotide. Lipid I modification with N-acetylglucosamine (GN) and pentaglycine cross bridge formation at the ɛ-amino of l-Lys with tRNAGly substrate generates lipid II. Following translocation across the cytoplasmic membrane, lipid II serves as substrate for PBPs that catalyze three reactions: transglycosylation, transpeptidation, and carboxypeptidation. Transglycosylases polymerize MN-GN subunits into repeating disaccharide chains, the glycan strands. Transpeptidases cleave the amide bond of the terminal d-Ala in pentapeptide precursors and generate an amide bond between the carboxyl group of d-Ala at position four and the amino group of pentaglycine cross bridges in wall peptides. Carboxypeptidases hydrolyze the C-terminal d-Ala of most non-cross-linked pentapeptides to yield mature peptidoglycan.