Figure 1.
Transport of Barley Phytepsin in Tobacco Protoplasts.
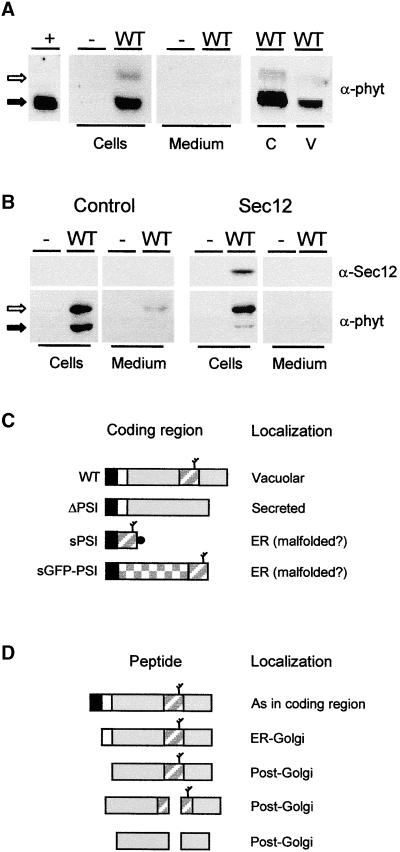
(A) Transient expression of wild-type Phytepsin (WT) in tobacco protoplasts. After transfection and incubation, equal quantities of cells and culture medium were loaded on SDS-PAGE gels for analysis by protein gel blotting using anti-Phytepsin serum (α-phyt). As a negative control, protoplasts were electroporated without DNA (−). As positive control, barley Phytepsin was extracted from barley grains (+). Note that the unprocessed form (open arrow) is not detected in the positive control, suggesting that in the steady state situation most of the Phytepsin is N-terminally processed (closed arrow). Phytepsin is detected in cell extracts, but it is not secreted into the culture medium. Cellular Phytepsin (C) is recovered from purified vacuoles (V). Extracts containing equal amounts of α-mannosidase (a vacuolar marker) were loaded in these two lanes.
(B) Similar to (A), but a threefold greater amount of plasmid carrying the gene encoding Phytepsin was transfected to saturate vacuolar targeting. The antisera used are indicated for Phytepsin (α-phyt) and Sec12p (α-Sec12). The right panel shows data from an experiment in which 10 μg of Sec12p-encoding plasmid was coelectroporated. Coexpression of Sec12p, which inhibits COPII vesicle budding from the ER (Phillipson et al., 2001), prevents the processing of cellular Phytepsin and secretion to the medium.
(C) Scheme of coding regions for Phytepsin and its derivatives. Signal peptides are shown in black, and propeptides are shown in white. The mature portion of Phytepsin is shown in gray, and the GFP is checkered. The PSI is indicated by a diagonally striped rectangle, and a branch indicates the glycan. The black circle indicates the myc epitope present on the PSI construct. WT, wild-type Phytepsin; ΔPSI, Phytepsin lacking the PSI domain; sPSI, soluble PSI; sGFP-PSI, secretory GFP fusion protein containing the PSI domain at the C terminus.
(D) Hypothetical processing pathway of barley Phytepsin expressed in tobacco protoplasts, based on findings past (Glathe et al., 1998) and present. Annotations are as in (C) and correspond only to wild-type Phytepsin. The fragmentation products formed in post-Golgi compartments are of low abundance in the steady state situation and cannot be detected in the exposures appropriate for the higher molecular weight forms.