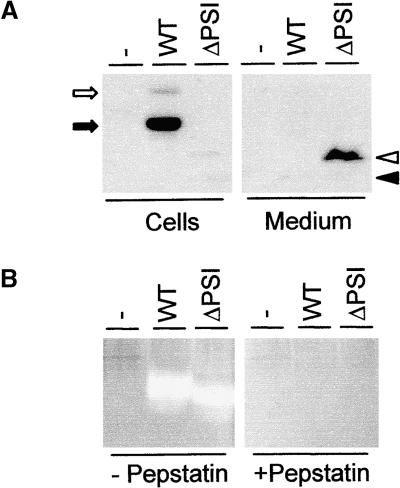
Figure 2.
Deletion of PSI Results in Secretion.
(A) Secretion/retention experiment using transient expression of wild-type Phytepsin (WT) and a truncated version lacking PSI (ΔPSI) in tobacco protoplasts. Annotations are as in Figure 1. Arrows indicate unprocessed (open arrow) and N-terminally processed (closed arrow) wild-type Phytepsin, whereas arrowheads indicate unprocessed (open arrowhead) and N-terminally processed (closed arrowhead) PhytepsinΔPSI. Note that the truncated protein, in contrast to the wild-type protein, is recovered mainly in the medium and that it is unprocessed.
(B) Proteinase activity assay showing that the PhytepsinΔPSI is proteolytically active. The presence (+) or absence (−) of pepstatin to specifically inhibit aspartic proteinase activity is indicated. Lanes from left to right are as follows: untransformed tobacco extract (−), extract from transgenic tobacco plant producing wild-type Phytepsin (WT), and extract from transgenic tobacco producing the PhytepsinΔPSI (ΔPSI). Note the strong bands of aspartic proteinase activity in both transgenic plant extracts, as shown by the appearance of nonstained regions.