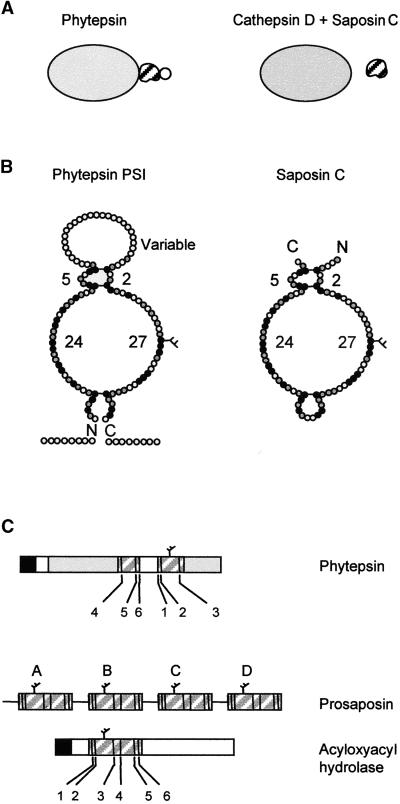
Figure 8.
Scheme of Phytepsin Compared with Mammalian Cathepsin D and Its Associated Partner Saposin C.
(A) Overall representation showing that the PSI is an integral part of Phytepsin, whereas different genes encode cathepsin D and saposin C. The variable domain of the PSI is indicated by a circle, which is not present in saposin C.
(B) Detailed diagram comparing PSI and saposin C showing the predicted structural similarity. Conserved amino acids are indicated in black and similar amino acids are shown in gray. The conserved disulfide bridges are indicated by horizontal bars. Numbers refer to the number of residues in each loop, which are conserved between the PSI of Phytepsin and saposin C. The C and N termini are indicated (C and N), and the conserved N-linked glycan is indicated by a branch.
(C) Comparison of the domain structure and order of appearance of the conserved cysteine residues (numbered according to saposin nomenclature) of the saposin-like motifs present in barley Phytepsin, the saposin precursor (prosaposin), and human acyloxyacyl hydrolase. Note that in Phytepsin, cysteines 1, 2, and 3 are displaced toward the C terminus of cysteines 4, 5, and 6 and are separated by the variable domain, which is unique to the plant sequence.