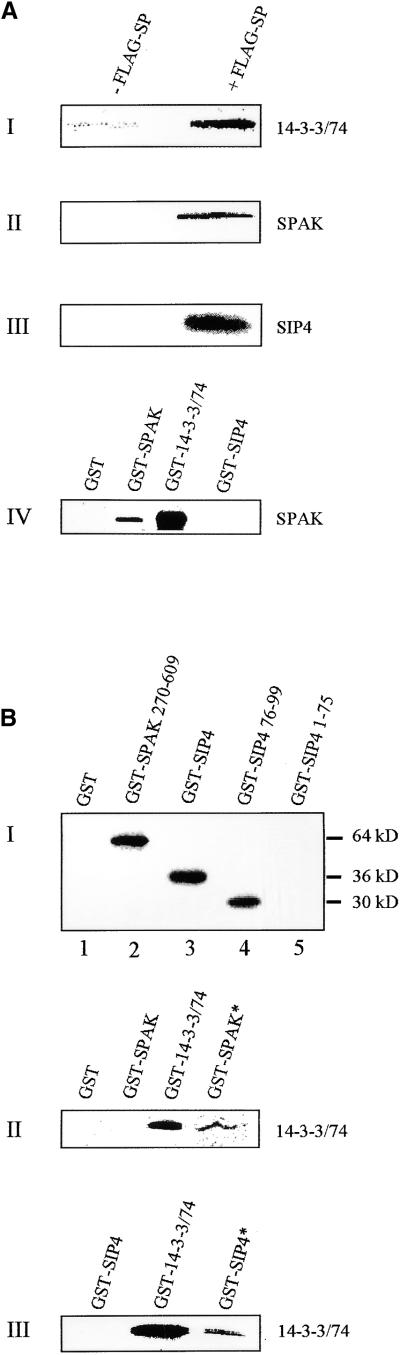
Figure 3.
SP and SIPs Interact in Vitro.
(A) In I to III, in vitro association of labeled 14-3-3/74, SPAK, and SIP4, respectively, to FLAG-SP protein is shown. The FLAG-SP gene was expressed in Sf9 cells. Proteins from expressing cells and control cells were immobilized on anti-FLAG agarose beads, incubated with 35S-Met–labeled SIPs, resolved on SDS-PAGE, and visualized by autoradiography. In IV, SPAK polypeptides form homodimers and interact in vitro with 14-3-3/74 but not with SIP4. Note that SPAK in IV, as well as SPAK and SIP4 in II and III, respectively, are phosphorylated.
(B) Phosphorylation-dependent associations between SIP proteins. In I, SPAK auto-phosphorylates and also phosphorylates the C-terminal region of SIP4. A GST fusion with the complete SPAK protein was incubated in a phosphorylation reaction assay with a GST control (lane 1); a GST-SPAK 270-609 protein lacking the kinase domain (60 kD; see Figure 6) (lane 2); a GST-SIP4 fusion protein (36 kD; amino acids 1 to 99) (lane 3); a GST-SIP4 76-99 (30 kD) (lane 4); and a GST-SIP4 1-75 N-terminal domain (lane 5). Proteins were separated by SDS-PAGE and autoradiographed. In II, 14-3-3/74 dimerizes and interacts with phosphorylated (*), but not with nonphosphorylated, GST-SPAK fusion protein. In III, 14-3-3/74 protein binds to GST-SIP4 only if the latter is phosphorylated. In both II and III, in vitro–translated 35S-Met–labeled SIPs (indicated to the right of each gel) were incubated with immobilized GST-SIP fusion proteins (indicated above each gel). The asterisks indicate a GST-SIP previously phosphorylated by SPAK. Samples of bound radioactive proteins were eluted, resolved on SDS-PAGE, and visualized by autoradiography.