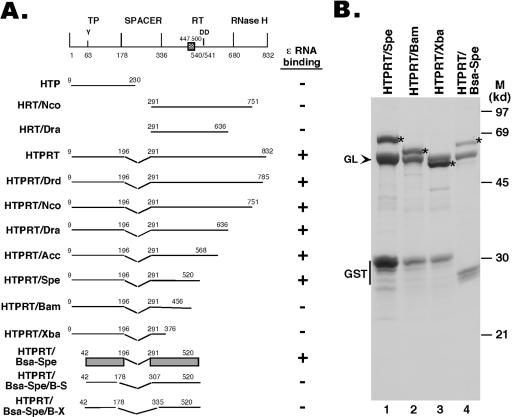
FIG. 1.
Expression of HBV RT truncation and deletion mutants. (A) Schematic diagram of the HBV RT proteins and domains expressed. Shown on the top is the domain structure of the HBV RT, with the primer tyrosine (Y63) and the double aspartate (D540/D541) in the RT active site denoted. The cross-hatched box denotes an insertion sequence (residues 447 to 500) in the HBV RT (and other mammalian RTs) relative to the DHBV RT, based on sequence alignment. The ends of the truncations and deletions are indicated. The ɛ binding activities of the RT mutants are summarized to the right. The shaded bars represented in the diagram for construct HTPRT/Bsa-Spe denote the boundaries of the TP and RT domains required for ɛ binding in vitro. (B) The truncated HBV RT proteins and domains were expressed as GST fusion proteins in bacteria, purified using glutathione resins, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and detected by Coomassie blue staining. The intact fusion proteins are indicated by asterisks. The bacterial chaperone protein GroEL (GL), copurifying with the RT proteins, is indicated by an arrowhead. The major degradation product, GST, is also indicated. M, molecular mass markers. Note that the first seven constructs in panel A have been reported recently (21).