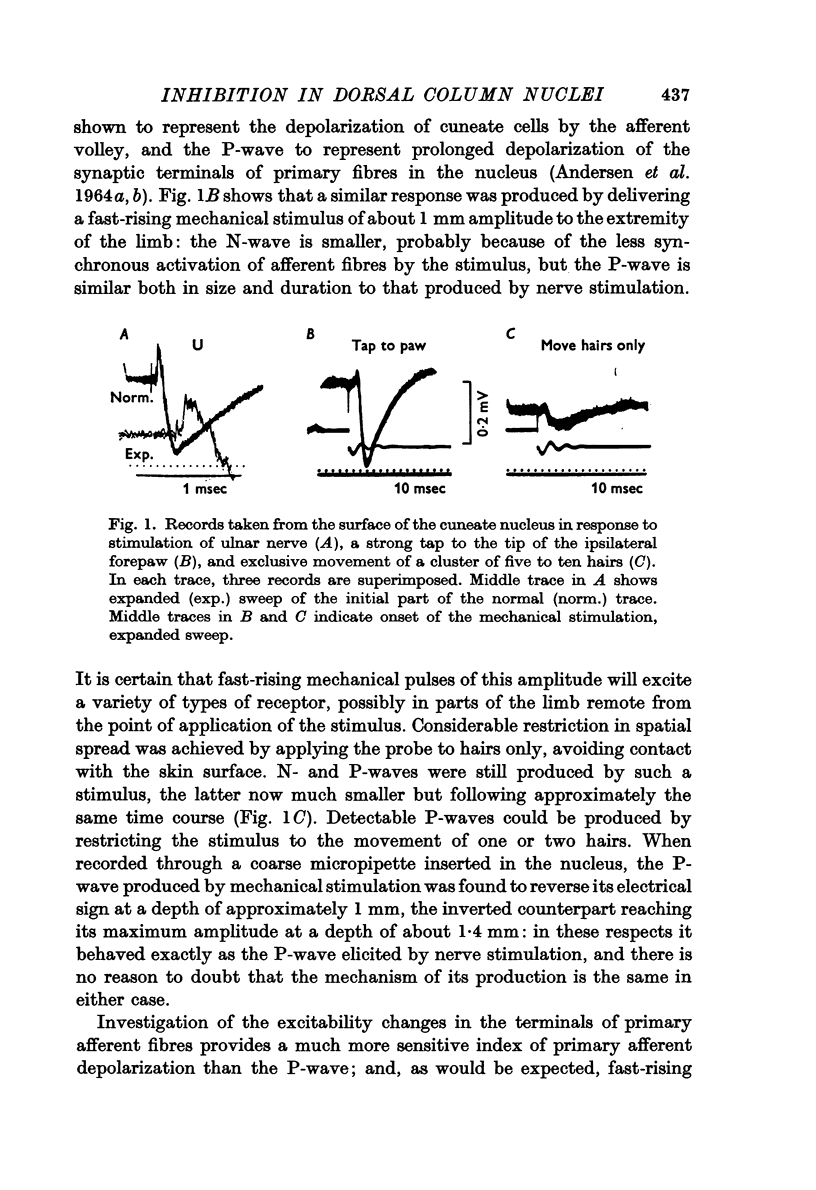
Abstract
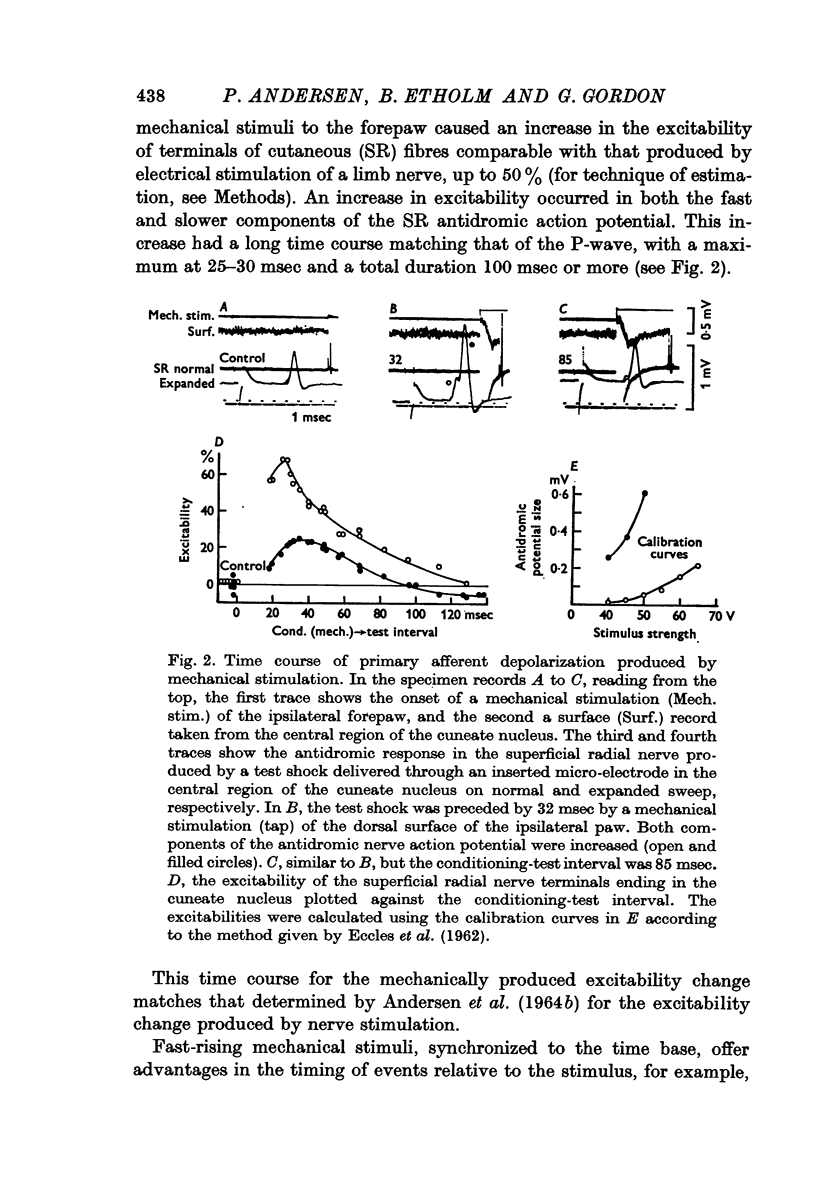
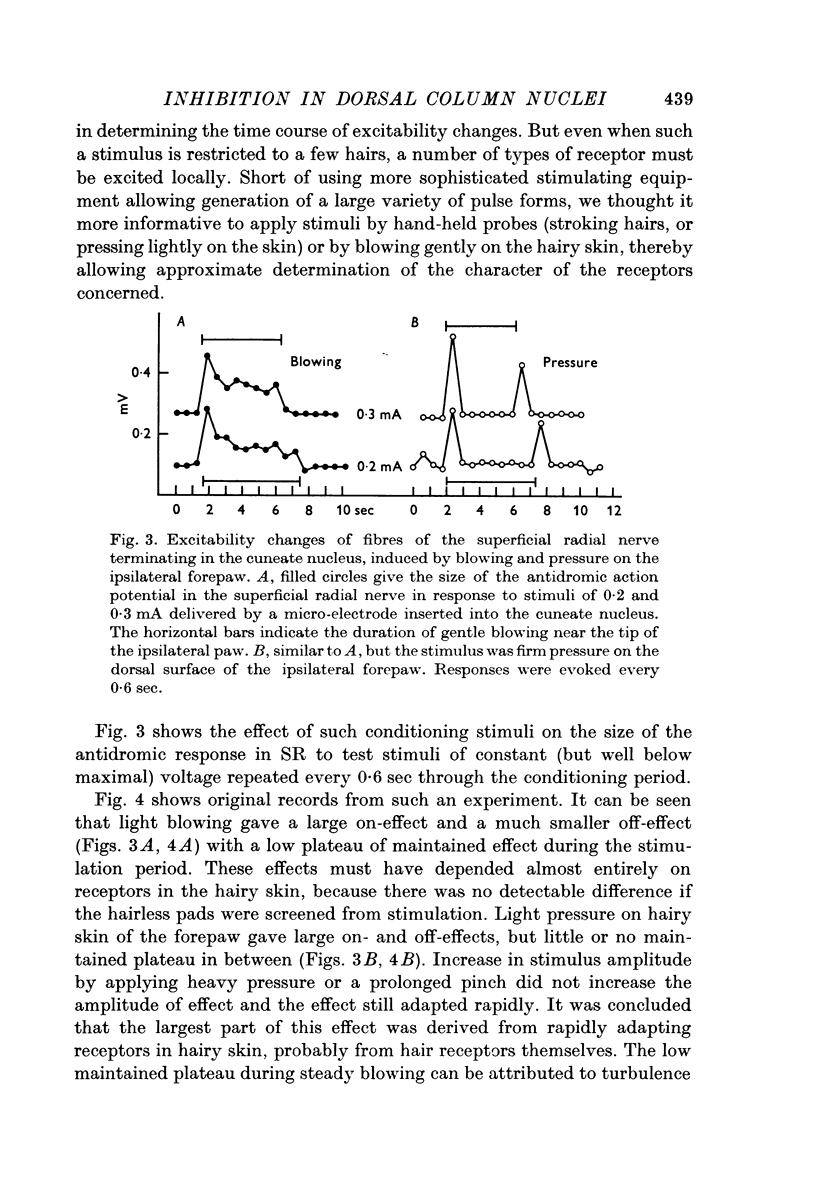
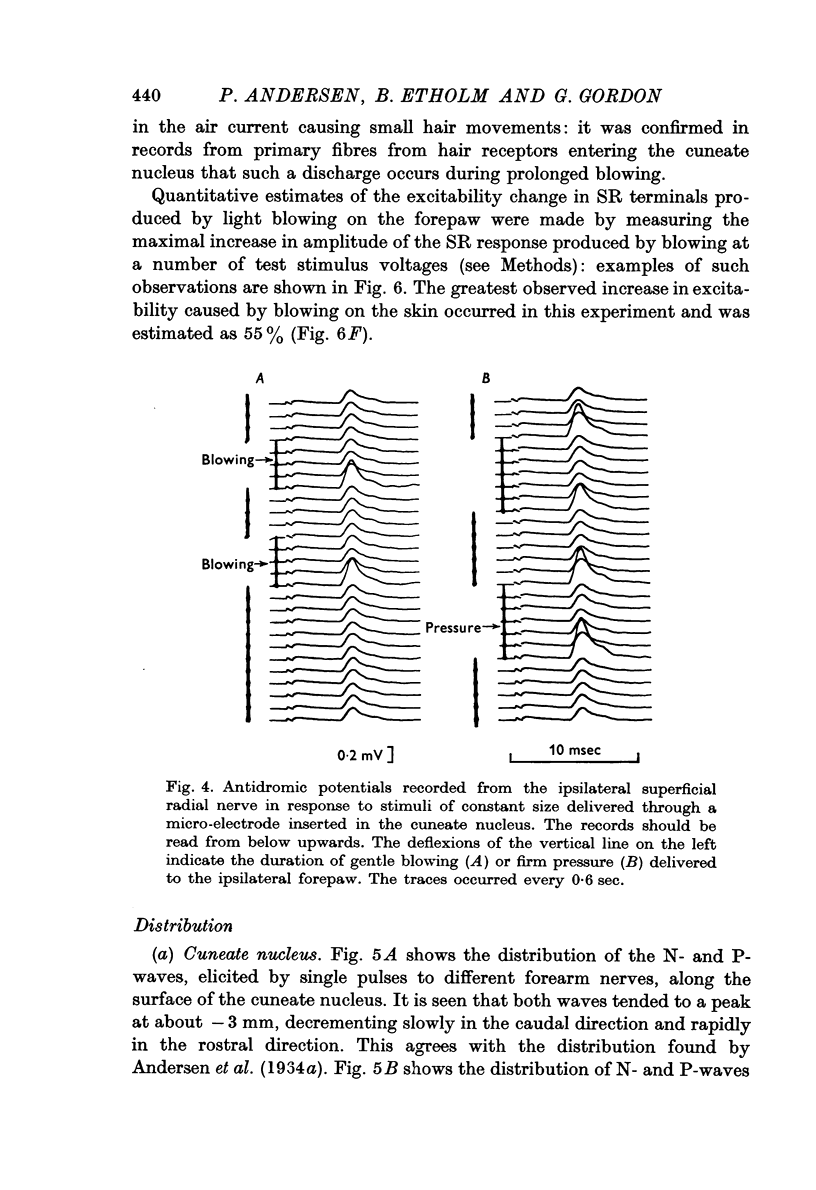
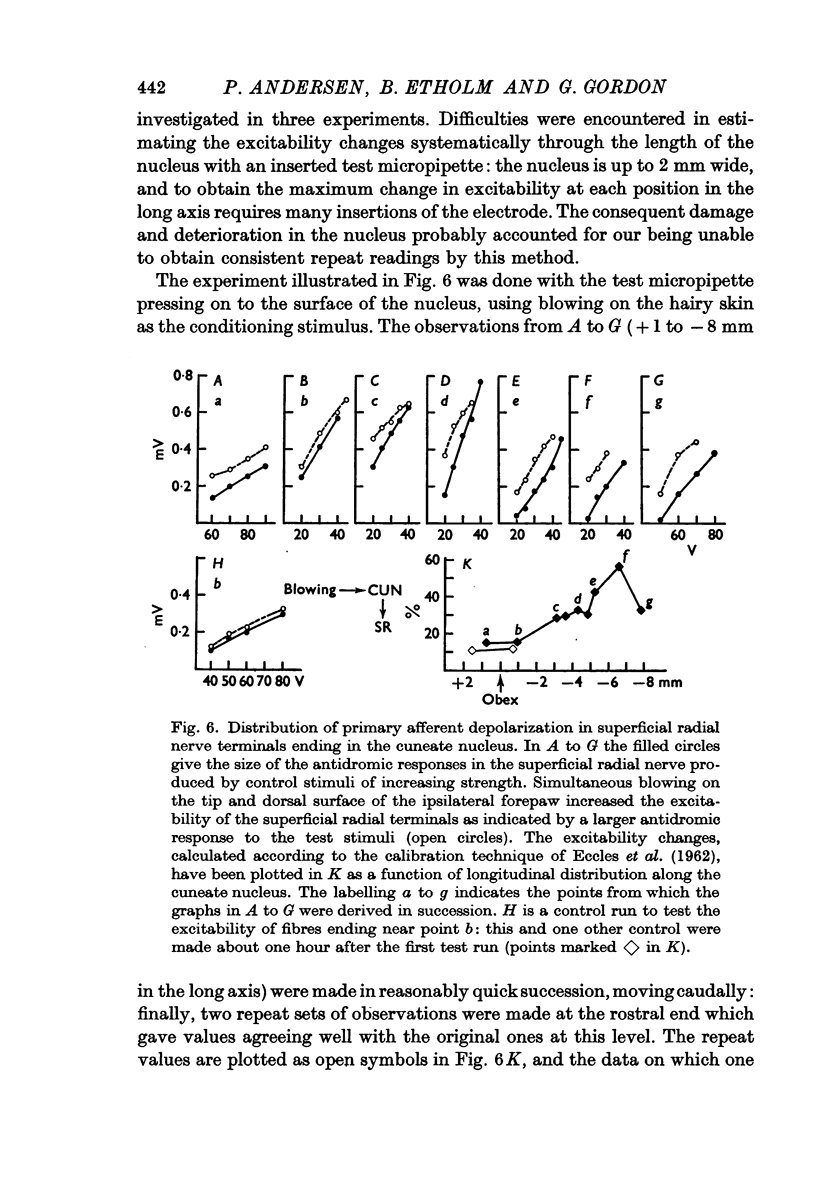
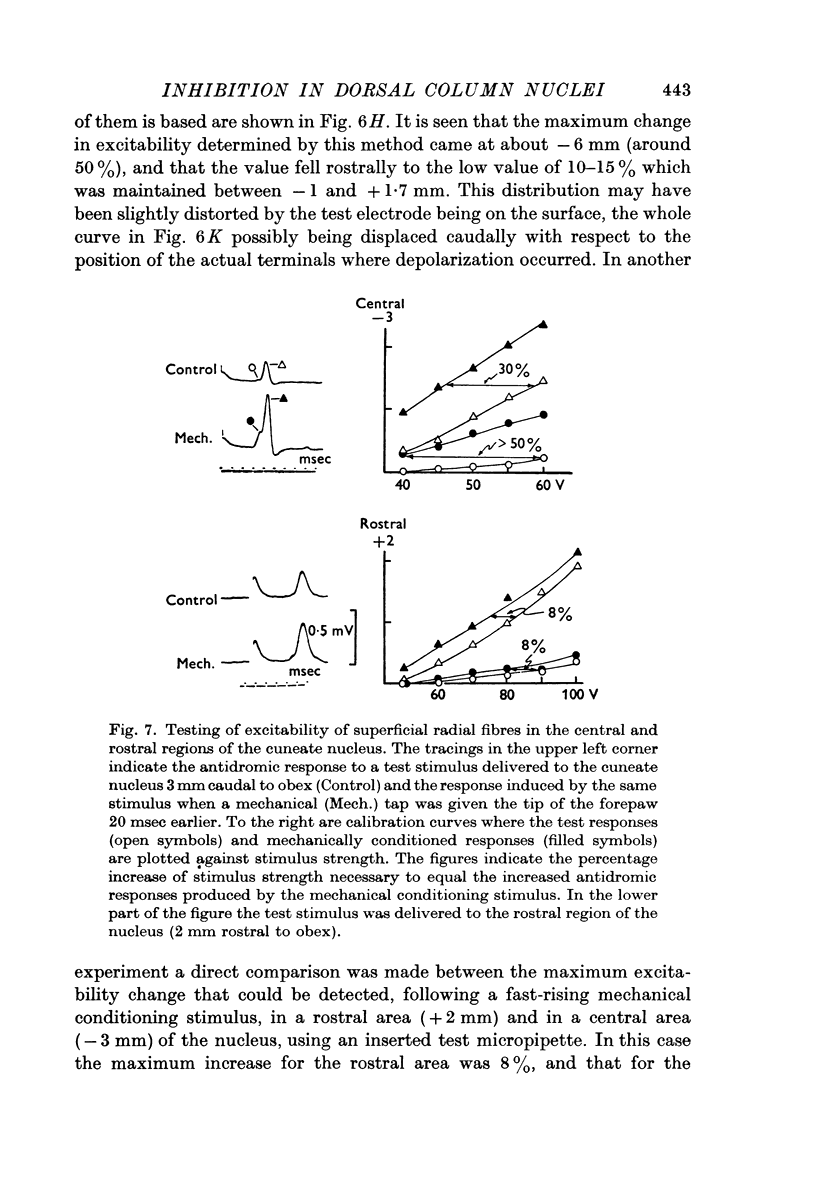
1. Primary afferent depolarization (PAD), with a time course comparable with that of the PAD following limb nerve stimulation, was produced in the cuneate nucleus by mechanical stimulation of the skin of the ipsilateral forepaw. Brushing or blowing on hairs was as effective as any other form of stimulation and there was a rapid adaptation to a sustained stimulus. Up to 55% increase in excitability was produced by blowing on hairs.
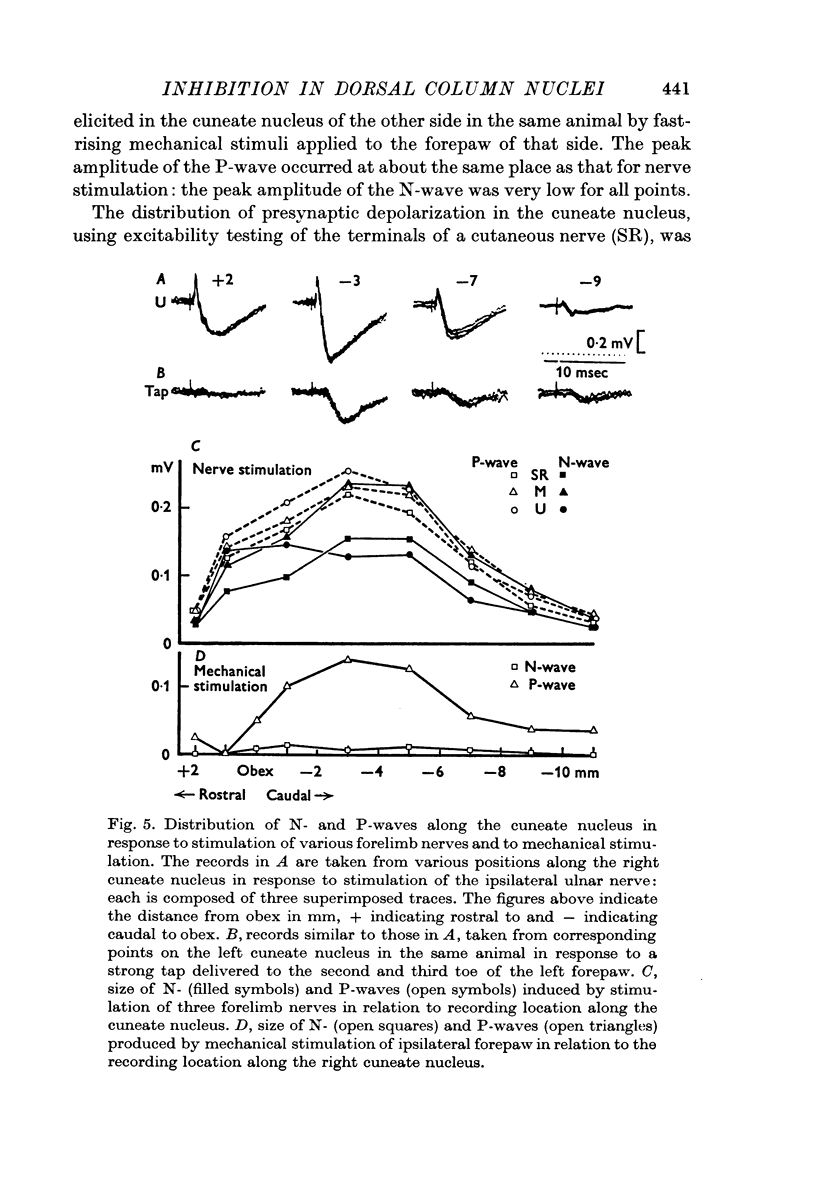
2. The P-wave and the PAD produced by mechanical stimulation were at a minimum in the rostral part of the cuneate nucleus and at a maximum 2-6 mm caudal to the obex.
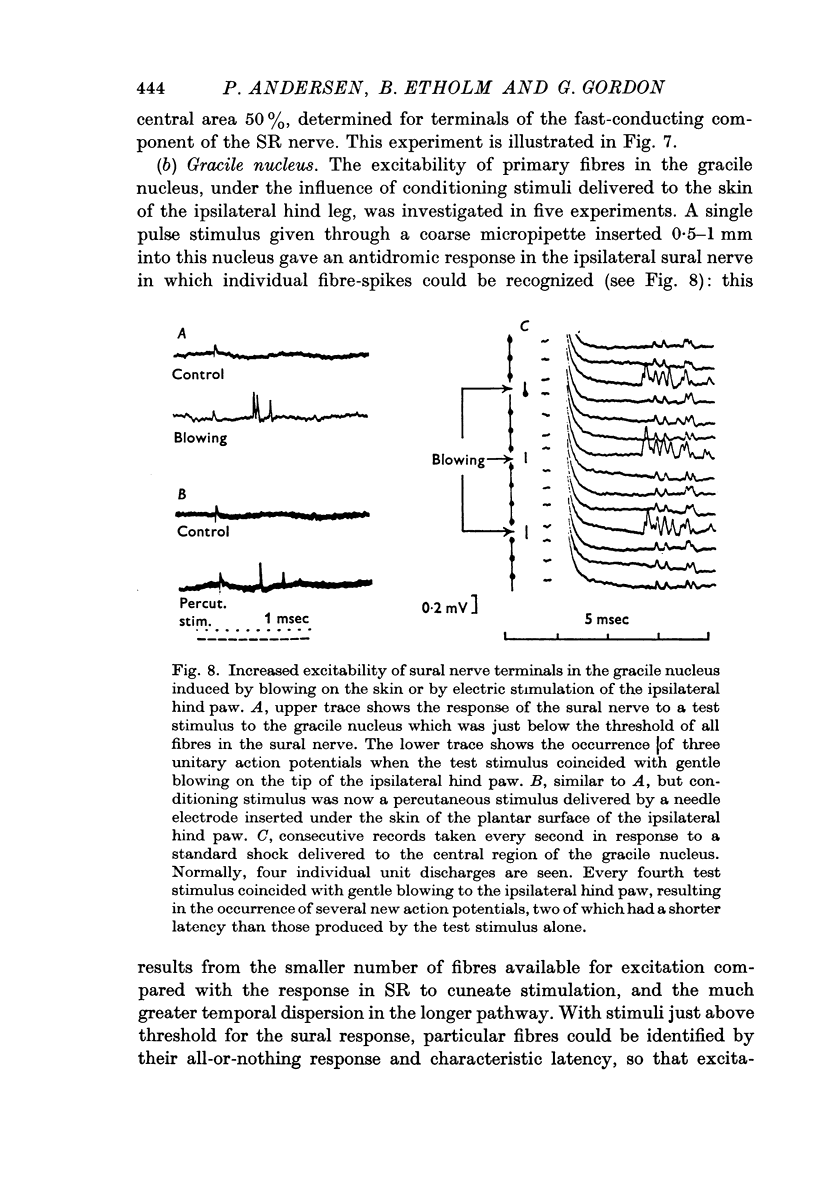
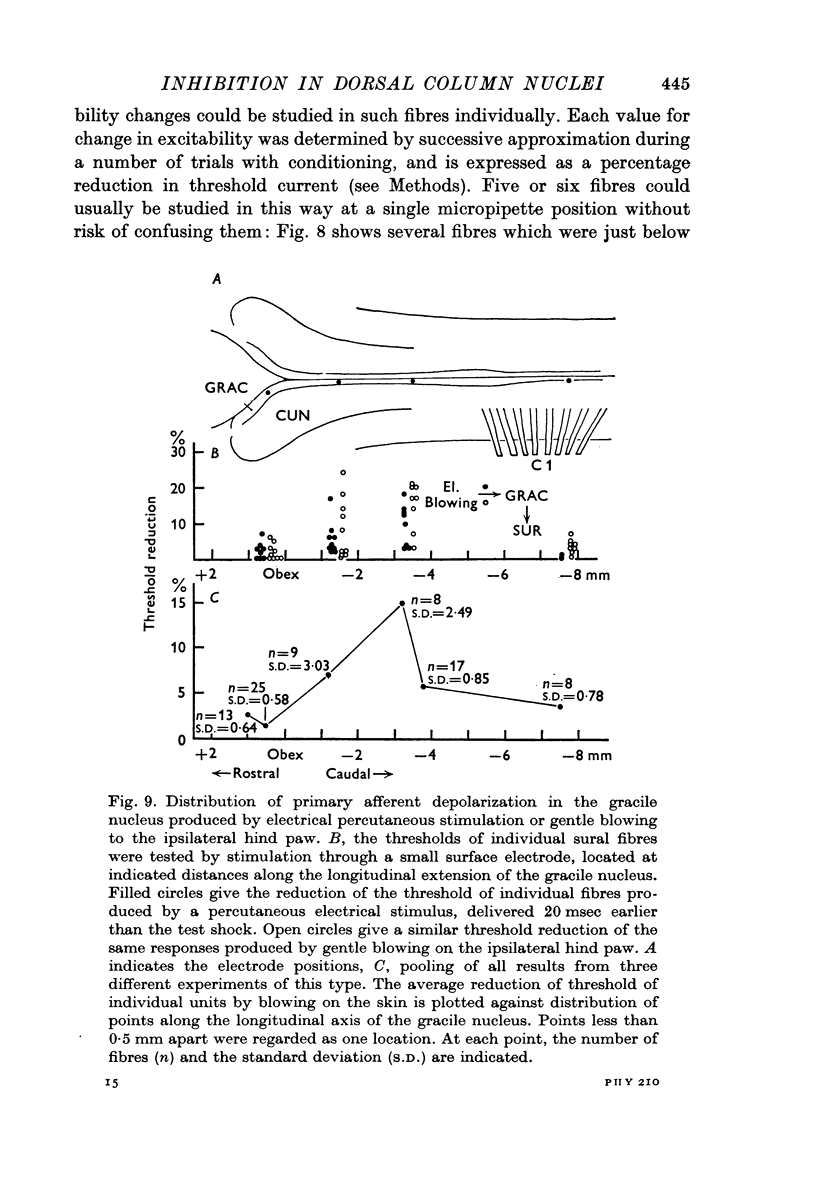
3. The distribution of the PAD produced in the gracile nucleus by blowing on the skin of the hind foot was studied by a technique allowing measurement of excitability changes near the terminals of single fibres. Minimal values were obtained rostral to the obex and maximal values 1-4 mm caudal to the obex, a distribution matching that previously determined for single cells subject to surround inhibition.
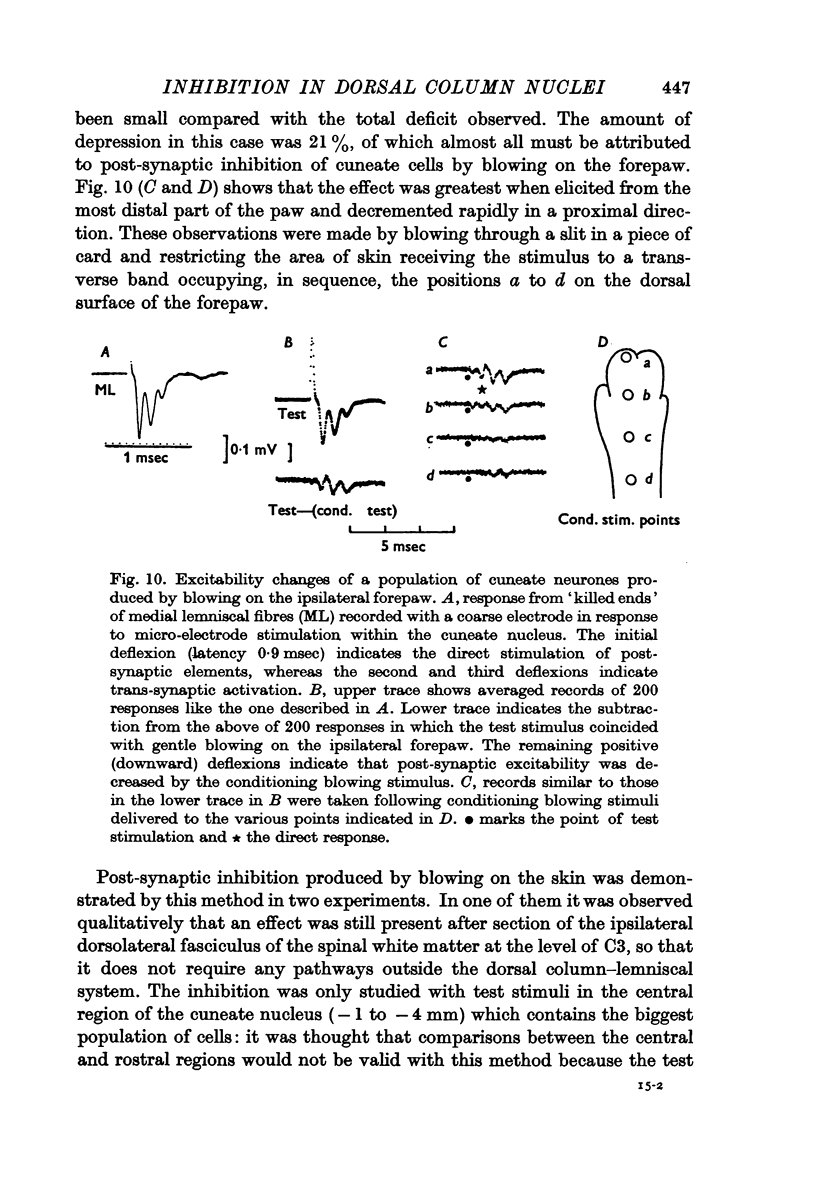
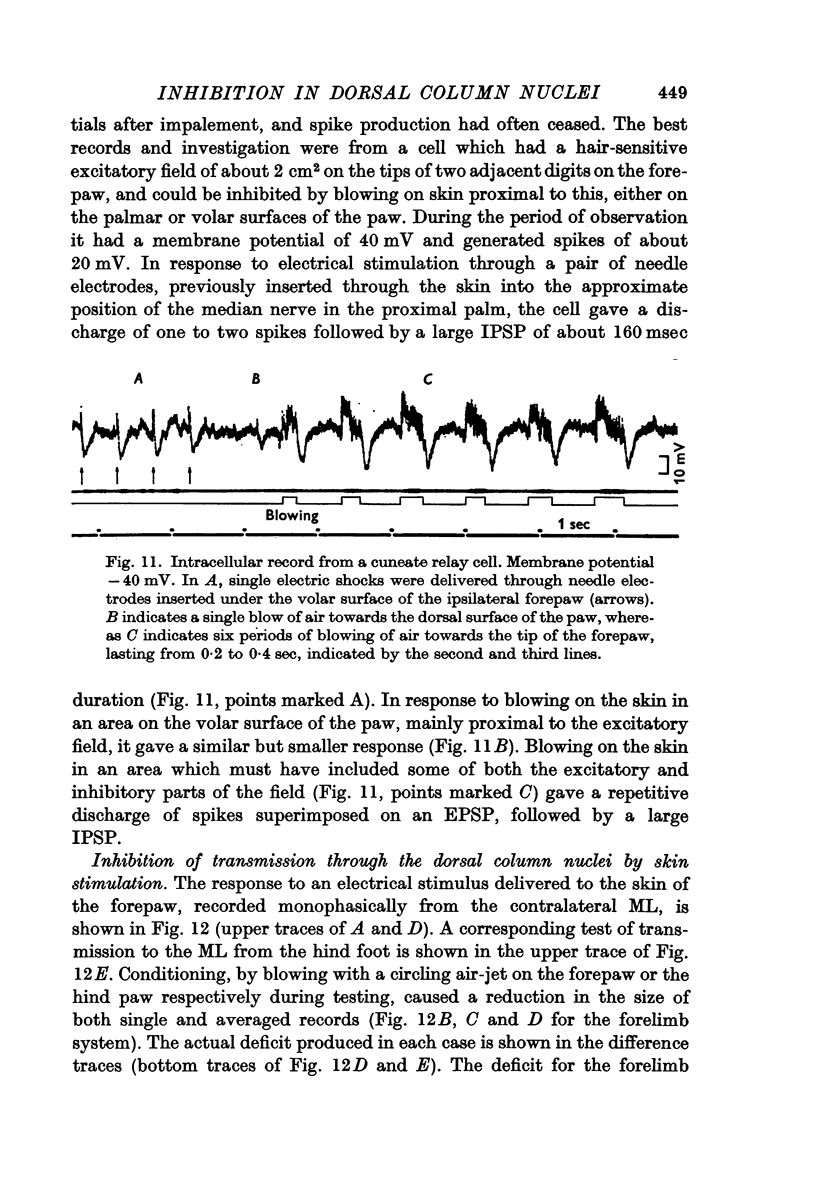
4. Post-synaptic inhibition was produced in the cuneate nucleus by gentle blowing on the ipsilateral forepaw. Up to 20% fall in excitability occurred in populations of cells tested by direct electrical stimulation. IPSPs lasting up to 160 msec were observed in single cells following light mechanical stimulation in the immediate neighbourhood of these cells' receptive fields. Blocking of antidromic invasion of single cells was occasionally produced by mechanical skin stimulation.
5. It is concluded that both pre- and post-synaptic inhibition must be concerned in the phenomenon of afferent surround inhibition, though there was no evidence to indicate their relative roles, qualitatively or quantitatively.
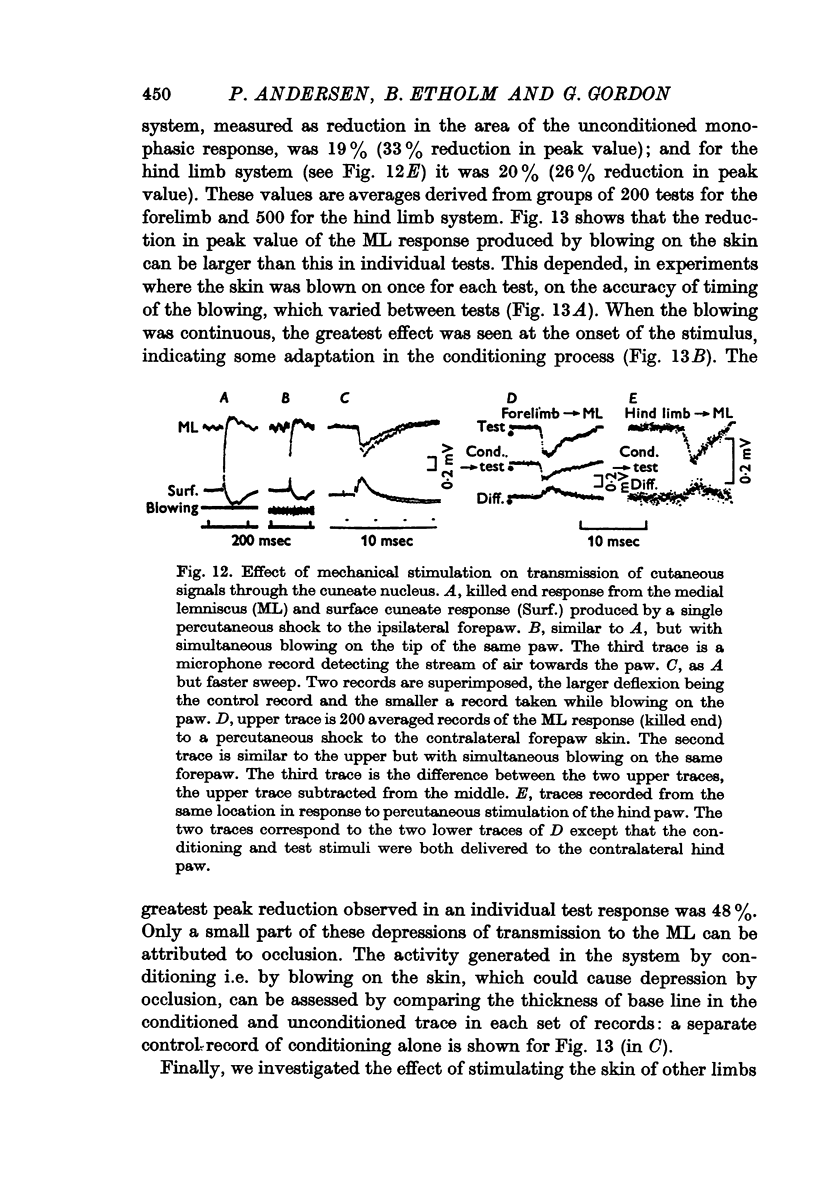
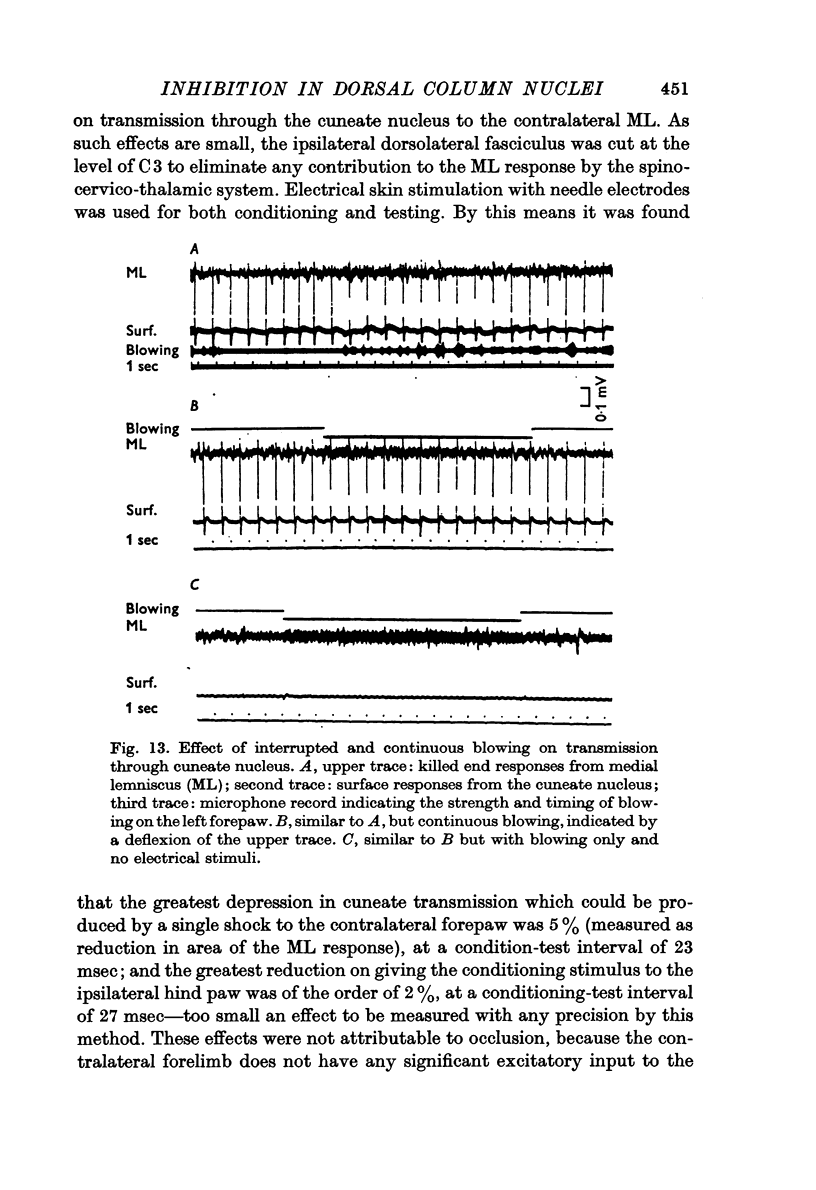
6. It is shown that up to 20% reduction in transmission through the gracile or cuneate nucleus could be produced by blowing on the ipsilateral hind paw or forepaw respectively, measured as reduction in the area of the monophasically recorded lemniscal response. A single electrical stimulus to the skin of the contralateral forepaw reduced transmission through the cuneate nucleus by less than 5%.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. DEPOLARIZATION OF PRESYNAPTIC FIBERS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Jan;27:92–106. doi: 10.1152/jn.1964.27.1.92. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. SLOW POTENTIAL WAVES PRODUCED IN THE CUNEATE NUCLEUS BY CUTANEOUS VOLLEYS AND BY CORTICAL STIMULATION. J Neurophysiol. 1964 Jan;27:78–91. doi: 10.1152/jn.1964.27.1.78. [DOI] [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic depolarization of dorsal column fibres by adequate stimulation. J Physiol. 1968 Feb;194(2):83–4P. [PubMed] [Google Scholar]
- DARIAN-SMITH I. PRESYNAPTIC COMPONENT IN THE AFFERENT INHIBITION OBSERVED WITHIN TRIGEMINAL BRAIN-STEM NUCLEI OF THE CAT. J Neurophysiol. 1965 Jul;28:695–709. doi: 10.1152/jn.1965.28.4.695. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedina L., Gordon G., Lundberg A. The source and mechanisms of inhibition in the lateral cervical nucleus of the cat. Brain Res. 1968 Dec;11(3):694–696. doi: 10.1016/0006-8993(68)90160-1. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., PAINE C. H. Functional organization in nucleus gracilis of the cat. J Physiol. 1960 Sep;153:331–349. doi: 10.1113/jphysiol.1960.sp006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbur S. J., Banna N. R. Presynaptic inhibition of cuneate transmission by widespread cutaneous inputs. Brain Res. 1968 Aug 26;10(2):273–276. doi: 10.1016/0006-8993(68)90135-2. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res. 1968;6(2):116–129. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G., TUERK J. D. THE DISTRIBUTION OF THE CORTICAL FIBRES WITHIN THE NUCLEI CUNEATUS AND GRACILIS IN THE CAT. J Anat. 1964 Apr;98:143–162. [PMC free article] [PubMed] [Google Scholar]
- PERL E. R., WHITLOCK D. G., GENTRY J. R. Cutaneous projection to second-order neurons of the dorsal column system. J Neurophysiol. 1962 May;25:337–358. doi: 10.1152/jn.1962.25.3.337. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F., Senges J., Zimmermann M. Excitability measurements at the central terminals of single mechano-receptor afferents during slow potential changes. Exp Brain Res. 1967;3(3):220–233. doi: 10.1007/BF00235586. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F., Senges J., Zimmermann M. Presynaptic depolarization of cutaneous mechanoreceptor afferents after mechanical skin stimulation. Exp Brain Res. 1967;3(3):234–247. doi: 10.1007/BF00235587. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]


