Abstract
1. Presynaptic fibres innervating the adult pigeon ciliary ganglion were cut 2 mm proximal to the ganglion. The modification of transmission in the ciliary and choroid cell populations was studied after periods of 6 hr—100 days.
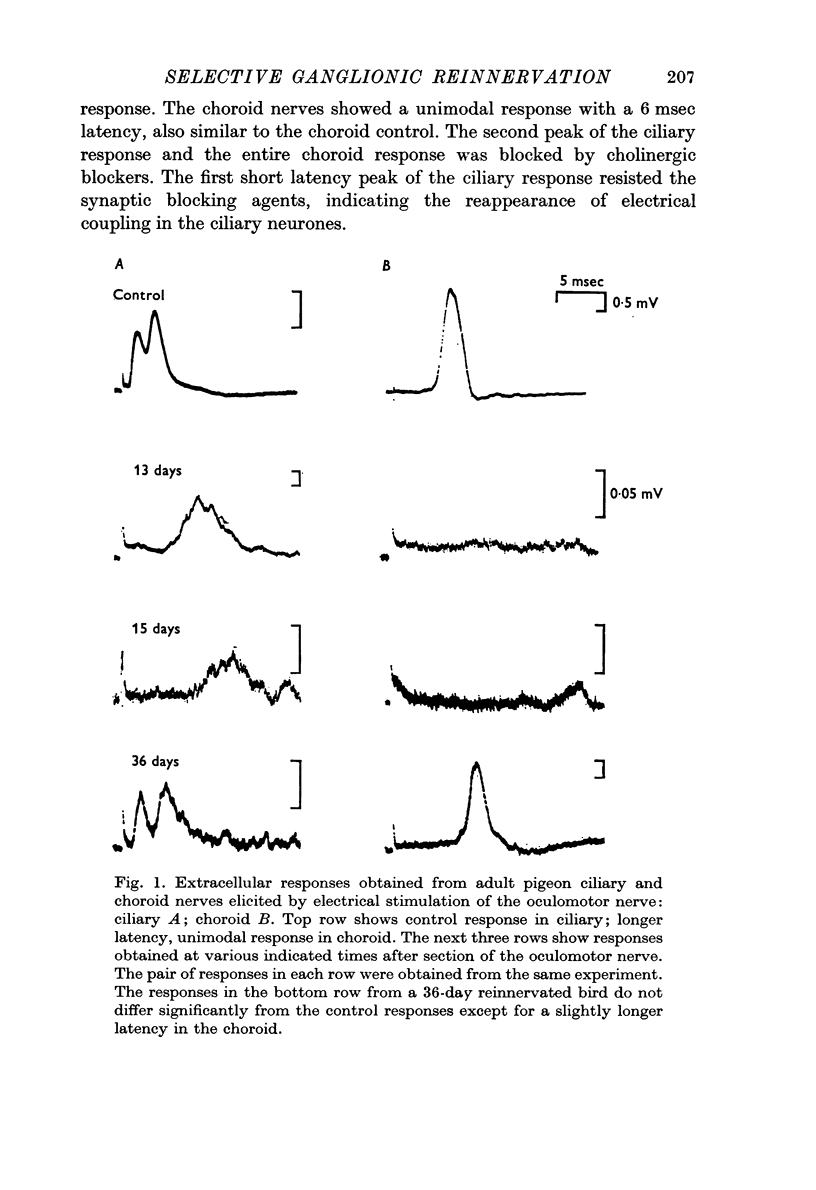
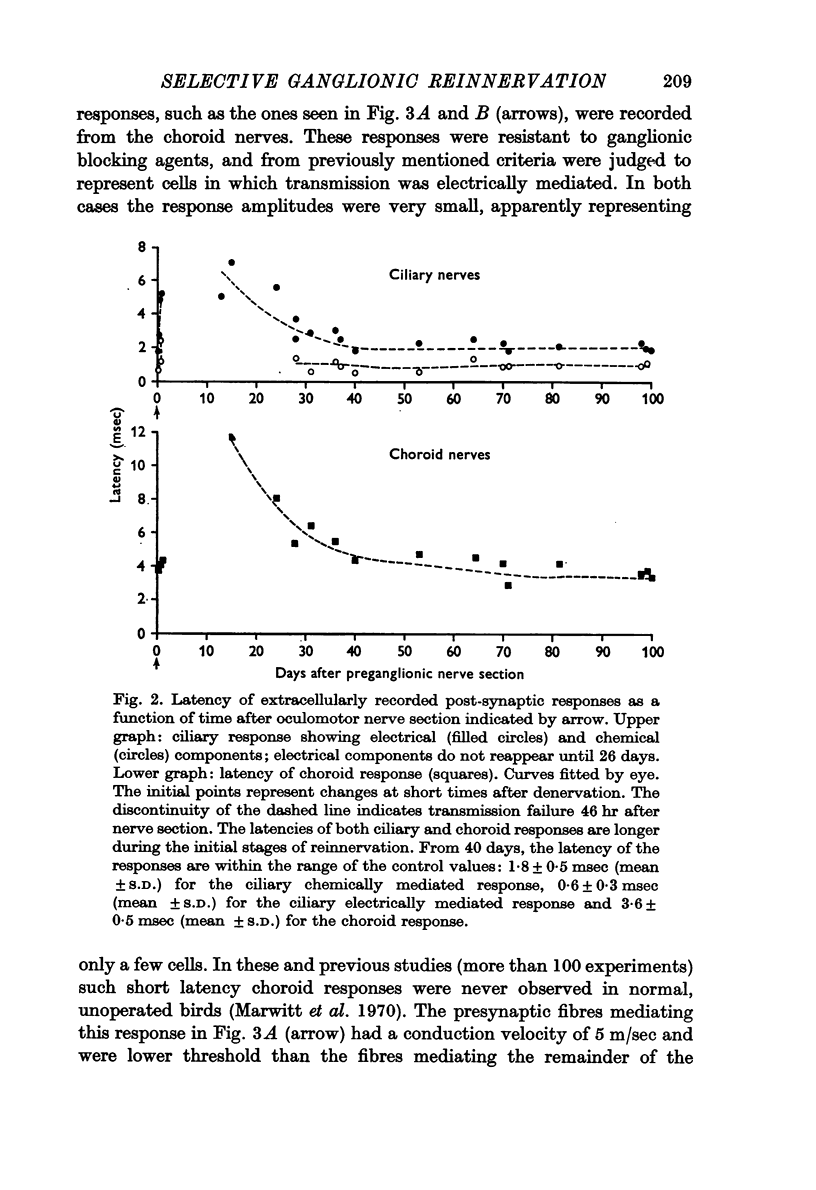
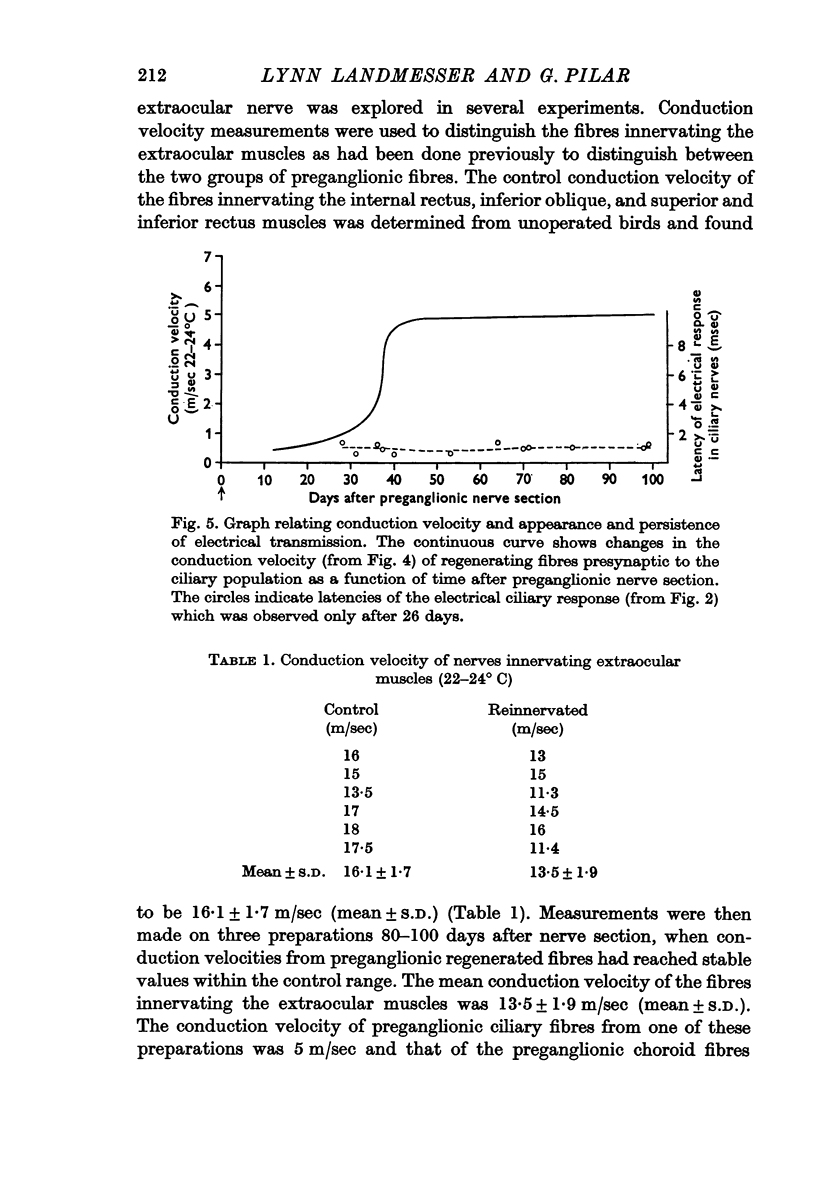
2. Transmission failed after 2 days and for the next 10 days there was no transmission through the ganglion. Long latency responses in both ciliary and choroid nerves were first observed at 13-15 days, and the latency decreased toward control values in 40 days. Electrical transmission reappeared in the ciliary population in about 26 days.
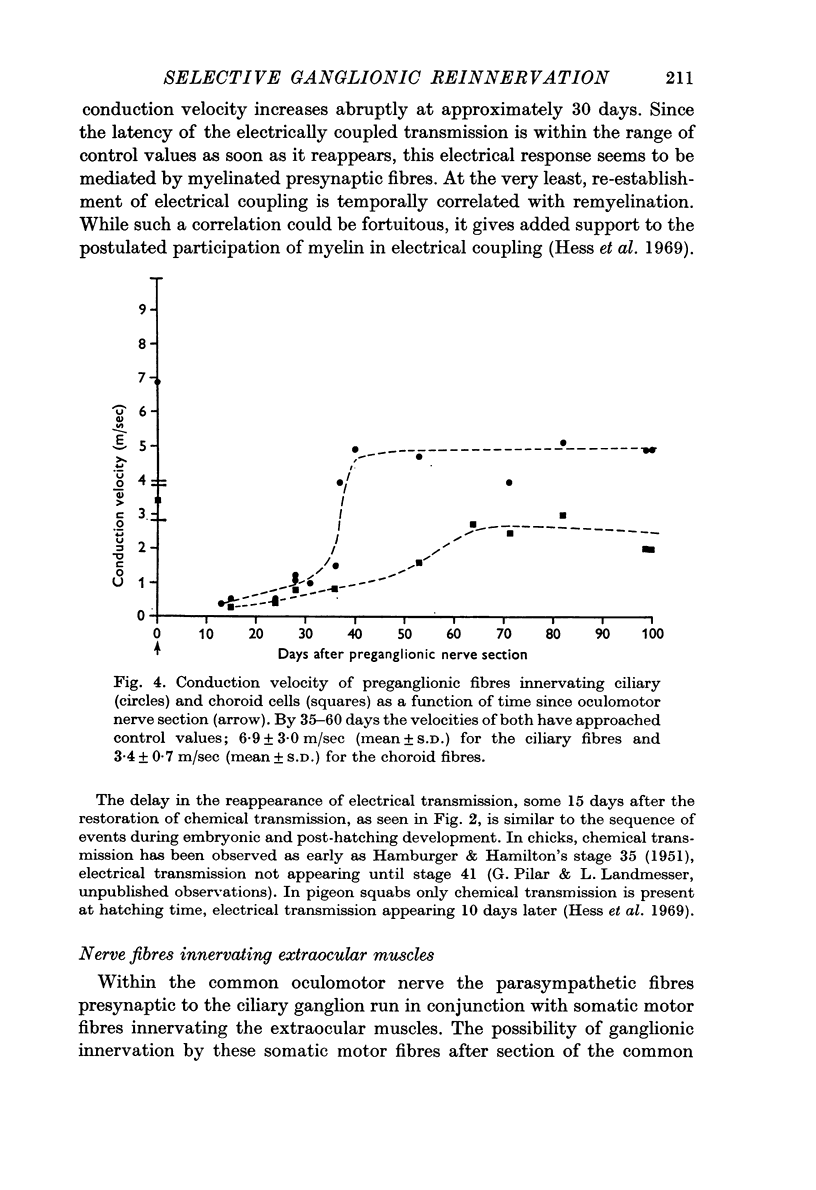
3. Presynaptic fibres innervating the ciliary population have higher conduction velocities and lower electrical thresholds than those innervating the choroid group. This relation was maintained throughout the reinnervation process. Fibres innervating the extraocular muscles also regenerated and achieved conduction velocities similar to their control values.
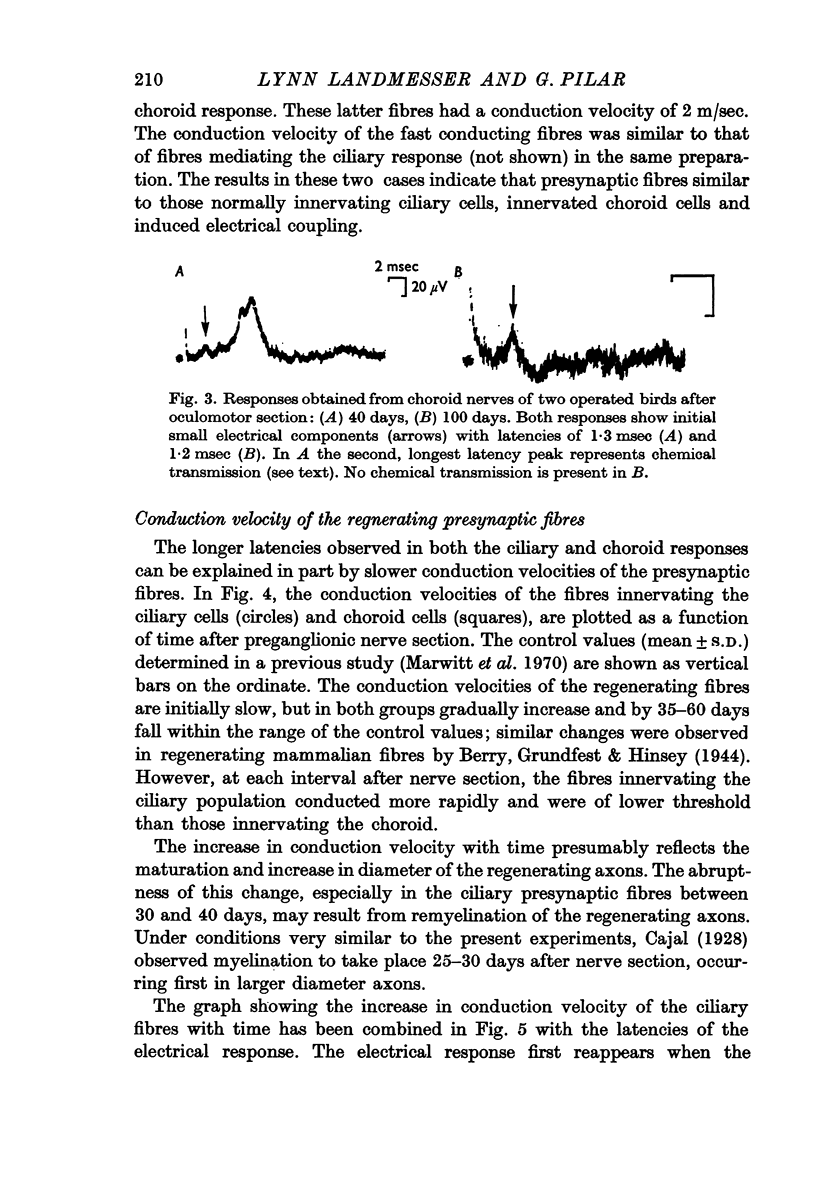
4. In two experiments out of seventeen, a few fast conducting, low threshold fibres, presumably stray ciliary fibres, innervated choroid cells and induced electrical transmission.
5. It is concluded that each group of cells, ciliary and choroid, was reinnervated in a highly selective manner by its original class of presynaptic fibres, and that the presynaptic ciliary elements cause the specializations necessary for electrical transmission.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN J. J., GUTH L. Nonselectivity in establishment of neuromuscular connections following nerve regeneration in the rat. Exp Neurol. 1961 Sep;4:262–275. doi: 10.1016/0014-4886(61)90047-4. [DOI] [PubMed] [Google Scholar]
- Bateman R. G., Rowley D. The natural resistance of pigeons to type 3 pneumococcus. Aust J Exp Biol Med Sci. 1969 Dec;47(6):733–745. doi: 10.1038/icb.1969.170. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Effects of nerve cross-union on fast-twitch and slow-graded muscle fibres in the toad. J Physiol. 1968 Sep;198(1):103–125. doi: 10.1113/jphysiol.1968.sp008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTH L., BERNSTEIN J. J. Selectivity in the re-establishment of synapses in the superior cervical sympathetic ganglion of the cat. Exp Neurol. 1961 Jul;4:59–69. doi: 10.1016/0014-4886(61)90078-4. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Murray M. Transport of protein in goldfish optic nerve during regeneration. Exp Neurol. 1969 Dec;25(4):494–508. doi: 10.1016/0014-4886(69)90093-4. [DOI] [PubMed] [Google Scholar]
- Hess A., Pilar G., Weakly J. N. Correlation between transmission and structure in avian ciliary ganglion synapses. J Physiol. 1969 Jun;202(2):339–354. doi: 10.1113/jphysiol.1969.sp008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the Regeneration of Pre-Ganglionic and of Post-Ganglionic Visceral Nerve Fibres. J Physiol. 1897 Nov 20;22(3):215–230. doi: 10.1113/jphysiol.1897.sp000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the Union of Cranial Autonomic (Visceral) Fibres with the Nerve Cells of the Superior Cervical Ganglion. J Physiol. 1898 Jul 26;23(3):240–270. doi: 10.1113/jphysiol.1898.sp000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. AN ANALYSIS OF ELECTRICAL COUPLING AT SYNAPSES IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Jun;171:454–475. doi: 10.1113/jphysiol.1964.sp007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Casella C. Synaptically mediated potentials elicited by the stimulation of post-ganglionic trunks in the guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):55–67. doi: 10.1007/BF00587046. [DOI] [PubMed] [Google Scholar]
- Pilar G., Vaughan P. C. Electrophysiological investigations of the pigeon iris neuromuscular junctions. Comp Biochem Physiol. 1969 Apr;29(1):51–72. doi: 10.1016/0010-406x(69)91725-3. [DOI] [PubMed] [Google Scholar]
- Pilar G., Vaughan P. C. Mechanical responses of the pigeon iris muscle fibres. Comp Biochem Physiol. 1969 Apr;29(1):73–87. doi: 10.1016/0010-406x(69)91726-5. [DOI] [PubMed] [Google Scholar]
- VERA C. L., VIAL J. D., LUCO J. V. Reinnervation of nictitating membrane of cat by cholinergic fibers. J Neurophysiol. 1957 Jul;20(4):365–373. doi: 10.1152/jn.1957.20.4.365. [DOI] [PubMed] [Google Scholar]
- WINDLE W. F. Regeneration of axons in the vertebrate central nervous system. Physiol Rev. 1956 Oct;36(4):427–440. doi: 10.1152/physrev.1956.36.4.427. [DOI] [PubMed] [Google Scholar]


